Rice husks, as a residue from agriculture, had been largely used as a source of power through direct incineration in major rice-producing countries. However, rice husks present an intriguing opportunity as a renewable source of SiO2, offering a low-cost adsorbent with a high surface area and ease of functionalization that can be transformed into diverse mesoporous silica structures or composites, enabling applications in catalysis, drug delivery, water treatment, etc. This dual potential of rice husks can be harnessed by combining bio-oil and syngas production through pyrolysis with the efficient extraction of SiO2, ensuring the comprehensive utilization of the biomass.
- SiO2 nanoparticles
- rice husk
- bio-oil
- circular economy
- biomass
- pyrolysis
1. Introduction
Rice Husk as a Source of Bio-Energy and SiO2
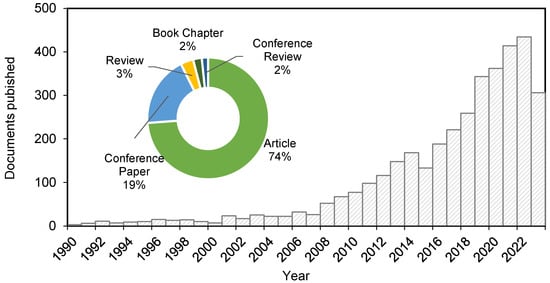
2. Sustainable Harnessing of SiO2 from Rice Husks
2.1. Traditional Thermochemical Methods: Calcination
2.2. Pyrolysis of Rice Husk for Obtaining Bio-Oil and Bio-Silica in the Literature
2.2.1. Bio-Silica Production
2.2.2. Combined Bio-Silica and Bio-Oil Production
3. Tailoring of SiO2 from Rice Husk to Enhance Porosity
3.1. Mesoporous RH-SiO2 Nanoparticles
3.2. Ordered Mesoporous RH-SiO2 Nanoparticles
3.3. Shaping RH-SiO2 NPs into Macroporous Structures
4. RH-SiO2 Applications
4.1. RH-SiO2 for Biomedical Applications
4.2. RH-SiO2 for Energy Storage
4.3. RH-SiO2 as Catalyst Support
4.4. RH-SiO2 as Adsorbent for Water Cleaning
4.4.1. Adsorption of Chemicals of Emerging Concern and Other Micropollutants
4.4.2. Adsorption of Metals in Water
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/pr11123373
References
- Imperial College London. Sustainable Biomass Availability in the EU, to 2050. 2021. Available online: https://www.concawe.eu/publication/sustainable-biomass-availability-in-the-eu-to-2050/ (accessed on 27 September 2022).
- An, D.; Guo, Y.; Zhu, Y.; Wang, Z. A green route to preparation of silica powders with rice husk ash and waste gas. Chem. Eng. J. 2010, 162, 509–514.
- Ng, E.-P.; Awala, H.; Tan, K.-H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice husk. Microporous Mesoporous Mater. 2015, 204, 204–209.
- Zhang, H.; Ding, X.; Chen, X.; Ma, Y.; Wang, Z.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing d-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73.
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of Lignocellulose and Synthesis of Porous Silica Nanoparticles from Rice Husks: A Comprehensive Utilization of Rice Husk Biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259.
- Gebretatios, A.G.; Pillantakath, A.R.K.K.; Witoon, T.; Lim, J.-W.; Banat, F.; Cheng, C.K. Rice husk waste into various template-engineered mesoporous silica materials for different applications: A comprehensive review on recent developments. Chemosphere 2023, 310, 136843.
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329.
- Rao, G.R.; Sastry, A.R.K.; Rohatgi, P.K. Nature and reactivity of silica available in rice husk and its ashes. Bull. Mater. Sci. 1989, 12, 469–479.
- Chen, P.; Gu, W.; Fang, W.; Ji, X.; Bie, R. Removal of metal impurities in rice husk and characterization of rice husk ash under simplified acid pretreatment process. Environ. Prog. Sustain. Energy 2017, 36, 830–837.
- Lu, Q.; Yang, X.-L.; Zhu, X.-F. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198.
- Nawaz, S.; Jamil, F.; Akhter, P.; Hussain, M.; Jang, H.; Park, Y.-K. Valorization of lignocellulosic rice husk producing biosilica and biofuels—A review. J. Physics Energy 2023, 5, 012003.
- Mahmad-Toher, A.-S.; Govender, N.; Dorairaj, D.; Wong, M.-Y. Comparative evaluation on calcium silicate and rice husk ash amendment for silicon-based fertilization of Malaysian rice (Oryza sativa L.) varieties. J. Plant Nutr. 2021, 45, 1336–1347.
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354.
- Möller, K.; Kobler, J.; Bein, T. Colloidal suspensions of mercapto-functionalized nanosized mesoporous silica. J. Mater. Chem. 2007, 17, 624–631.
- Zhang, A.; Gu, L.; Hou, K.; Dai, C.; Song, C.; Guo, X. Mesostructure-tunable and size-controllable hierarchical porous silica nanospheres synthesized by aldehyde-modified Stöber method. RSC Adv. 2015, 5, 58355–58362.
- Lou, F.; Zhang, A.; Zhang, G.; Ren, L.; Guo, X.; Song, C. Enhanced kinetics for CO2 sorption in amine-functionalized mesoporous silica nanosphere with inverted cone-shaped pore structure. Appl. Energy 2020, 264, 114637.
- Akinjokun, A.I.; Ojumu, T.V.; Ogunfowokan, A.O. Biomass, Abundant Resources for Synthesis of Mesoporous Silica Material. In Microporous and Mesoporous Materials; BoD—Books on Demand: Norderstedt, Germany, 2016; pp. 105–177.
- James, J.; Rao, M. Silica from rice husk through thermal decomposition. Thermochim. Acta 1986, 97, 329–336.
- Kapur, P. Production of reactive bio-silica from the combustion of rice husk in a tube-in-basket (TiB) burner. Powder Technol. 1985, 44, 63–67.
- Salavati-Niasari, M.; Javidi, J.; Dadkhah, M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb. Chem. High Throughput Screen. 2013, 16, 458–462.
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885.
- Benassi, L.; Bosio, A.; Dalipi, R.; Borgese, L.; Rodella, N.; Pasquali, M.; Depero, L.E.; Bergese, P.; Bontempi, E. Comparison between rice husk ash grown in different regions for stabilizing fly ash from a solid waste incinerator. J. Environ. Manag. 2015, 159, 128–134.
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of organic acid treatment on the properties of rice husk silica. J. Mater. Sci. 2005, 40, 6535–6544.
- Liou, T.-H. Evolution of chemistry and morphology during the carbonization and combustion of rice husk. Carbon 2004, 42, 785–794.
- Wang, W.; Martin, J.C.; Zhang, N.; Ma, C.; Han, A.; Sun, L. Harvesting silica nanoparticles from rice husks. J. Nanoparticle Res. 2011, 13, 6981–6990.
- Liou, T.-H.; Yang, C.-C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater. Sci. Eng. B 2011, 176, 521–529.
- Hsu, C.-P.; Huang, A.-N.; Kuo, H.-P. Analysis of the Rice Husk Pyrolysis Products from a Fluidized Bed Reactor. Procedia Eng. 2015, 102, 1183–1186.
- Manasa, K.; Naresh, G.; Kalpana, M.; Sasikumar, B.; Velisoju, V.K.; Chary, K.V.; Michalkiewicz, B.; Venugopal, A. Improved H2 yields over rice husk derived SiO2 nanoparticles supported Ni catalyst during non-oxidative methane cracking. J. Energy Inst. 2021, 99, 73–81.
- Choudhary, P.; Sharma, R.; Kumar, V.; Singh, A.; Sharma, N. Synthesis, Characterization and Catalytic Activity of Bio-MCM-41 for Production of Bio Crude Oil via Pyrolysis of Rice Straw. Waste Biomass Valorization 2023, 14, 4173–4186.
- Alyosef, H.A.; Uhlig, H.; Münster, T.; Kloess, G.; Einicke, W.-D.; Gläser, R.; Enke, D. Biogenic silica from rice husk ash-Sustainable sources for the synthesis of value-added silica. Chem. Eng. Trans. 2014, 37, 667–672.
- Costa, J.A.S.; Sarmento, V.H.V.; Romão, L.P.C.; Paranhos, C.M. Adsorption of organic compounds on mesoporous material from rice husk ash (RHA). Biomass Convers. Biorefinery 2020, 10, 1105–1120.
- Kamari, S.; Ghorbani, F. Extraction of highly pure silica from rice husk as an agricultural by-product and its application in the production of magnetic mesoporous silica MCM–41. Biomass Convers. Biorefinery 2021, 11, 3001–3009.
- Bahrami, A.; Simon, U.; Soltani, N.; Zavareh, S.; Schmidt, J.; Pech-Canul, M.I.; Gurlo, A. Eco-fabrication of hierarchical porous silica monoliths by ice-templating of rice husk ash. Green Chem. 2017, 19, 188–195.
- Athinarayanan, J.; Periasamy, V.S.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram. Int. 2015, 41, 275–281.
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2021, 19, 1667–1691.
- Huang, S.-S.; Tung, M.T.; Huynh, C.D.; Hwang, B.-J.; Bieker, P.M.; Fang, C.-C.; Wu, N.-L. Engineering Rice Husk into a High-Performance Electrode Material through an Ecofriendly Process and Assessing Its Application for Lithium-Ion Sulfur Batteries. ACS Sustain. Chem. Eng. 2019, 7, 7851–7861.
- Vijayan, R.; Kumar, G.S.; Karunakaran, G.; Surumbarkuzhali, N.; Prabhu, S.; Ramesh, R. Microwave combustion synthesis of tin oxide-decorated silica nanostructure using rice husk template for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2020, 31, 5738–5745.
- Franco, A.; De, S.; Balu, A.M.; Romero, A.A.; Luque, R. Integrated Mechanochemical/Microwave-Assisted Approach for the Synthesis of Biogenic Silica-Based Catalysts from Rice Husk Waste. ACS Sustain. Chem. Eng. 2018, 6, 11555–11562.
- Madduluri, V.R.; Mandari, K.K.; Velpula, V.; Varkolu, M.; Kamaraju, S.R.R.; Kang, M. Rice husk-derived carbon-silica supported Ni catalysts for selective hydrogenation of biomass-derived furfural and levulinic acid. Fuel 2020, 261, 116339.
- An, D.; Guo, Y.; Zou, B.; Zhu, Y.; Wang, Z. A study on the consecutive preparation of silica powders and active carbon from rice husk ash. Biomass Bioenergy 2011, 35, 1227–1234.
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.A.; Jothirani, R. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalination Water Treat. 2019, 172, 395–416.
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608.
- Kollarahithlu, S.C.; Balakrishnan, R.M. Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. J. Clean. Prod. 2021, 294, 126155.
- Peralta, M.E.; Mártire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841.
- Soltani, R.D.C.; Khorramabadi, G.S.; Khataee, A.; Jorfi, S. Silica nanopowders/alginate composite for adsorption of lead (II) ions in aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 973–980.
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of copper (II) on mesoporous silica: The effect of nano-scale confinement. Geochem. Trans. 2018, 19, 13.
- Chen, W.; Zhang, H.; Liang, Y.; Ding, H.; Sun, S. Adsorption Properties and Mechanism of Cd2+ in Water by Zr-containing Silica Residue Purification. Front. Chem. 2018, 6, 556.
- Ebner, A.D.; Ritter, J.A.; Navratil, J.D. Adsorption of Cesium, Strontium, and Cobalt Ions on Magnetite and a Magnetite−Silica Composite. Ind. Eng. Chem. Res. 2001, 40, 1615–1623.
- Nguyen, T.T.; Ma, H.T.; Avti, P.; Bashir, M.J.K.; Ng, C.A.; Wong, L.Y.; Jun, H.K.; Ngo, Q.M.; Tran, N.Q. Adsorptive removal of iron using SiO2 nanoparticles extracted from rice husk ash. J. Anal. Methods Chem. 2019, 2019, 6210240.
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.; Rashwan, A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021, 28, 5119–5130. Available online: https://www.sciencedirect.com/science/article/pii/S1319562X21003958 (accessed on 6 December 2022).
- Sobhanardakani, S.; Parvizimosaed, H.; Olyaie, E. Heavy metals removal from wastewaters using organic solid waste—Rice husk. Environ. Sci. Pollut. Res. 2013, 20, 5265–5271.
- Priya, A.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796.
- Amirhandeh, S.Z.H.; Salem, A.; Salem, S. Treatment of tannery wastewater by silica nanoparticles produced from rice husk ash via a green route. Environ. Sci. Pollut. Res. 2022, 30, 13039–13047.
