Neuronal differentiation has been shown to be directed by retinoid action during embryo development and has been exploited in various in vitro cell differentiation systems. In this research, the researchers summarize the role of retinoids through the activation of their specific retinoic acid nuclear receptors during embryo development and also in a variety of in vitro strategies for neuronal differentiation, including efforts in driving cell specialization towards a range of neuronal subtypes and glial cells. Finally, the researchers highlight the role of retinoic acid in protocols recapitulating nervous tissue complexity (cerebral organoids). Overall, the researchers expect that this effort might pave the way for exploring the usage of specific synthetic retinoids for directing complex nervous tissue differentiation.
- stem cells
- neuronal differentiation
- retinoids
- cell specialization
1. Introduction
2. Retinoic Acid Uptake and Metabolism
3. Retinoic Acid Receptors
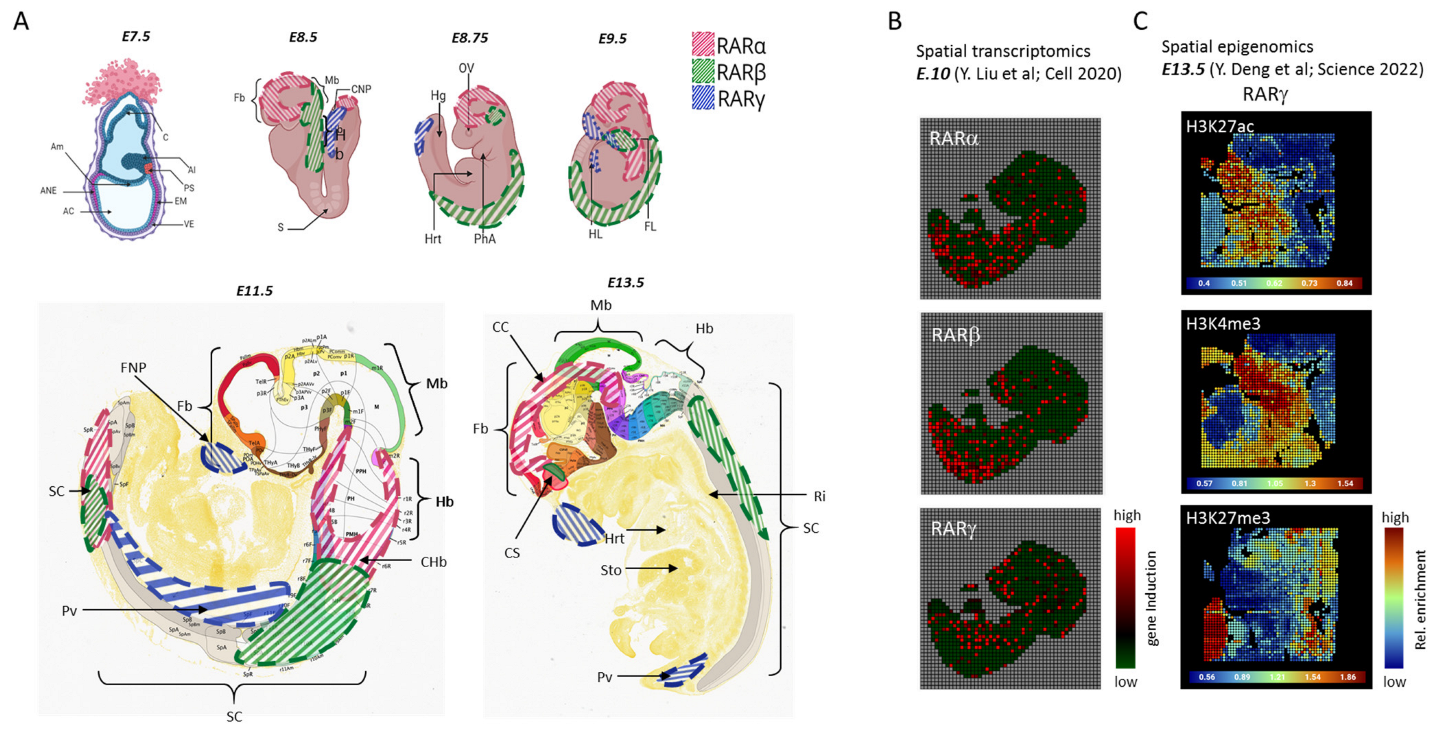
4. The Role and Distribution of Retinoic Acid in Embryogenesis
This entry is adapted from the peer-reviewed paper 10.3390/life13122279
References
- Semba, R.D. On the “discovery” of Vitamin A. Ann. Nutr. Metab. 2012, 61, 192–198.
- Hopkins, F.G. Feeding Experiments Illustrating the Importance of Accessory Factors in Normal Dietaries. J. Physiol. 1912, 44, 425–460.
- Wolbach, S.B.; Howe, P.R. Tissue Changes Following Deprivation of Fat-Soluble A Vitamin. J. Exp. Med. 1925, 42, 753–777.
- Giguère, V.; Evans, R.M. Chronicle of a Discovery: The Retinoic Acid Receptor. J. Mol. Endocrinol. 2022, 69, T1–T11.
- Blomhoff, R.; Blomhoff, H.K. Overview of Retinoid Metabolism and Function. J. Neurobiol. 2006, 66, 606–630.
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825.
- Duester, G. Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 2008, 134, 921–931.
- Ang, H.L.; Deltour, L.; Hayamizu, T.F.; Zgombić-Knight, M.; Duester, G. Retinoic Acid Synthesis in Mouse Embryos during Gastrulation and Craniofacial Development Linked to Class IV Alcohol Dehydrogenase Gene Expression. J. Biol. Chem. 1996, 271, 9526–9534.
- Deltour, L.; Foglio, M.H.; Duester, G. Metabolic Deficiencies in Alcohol Dehydrogenase Adh1, Adh3, and Adh4 Null Mutant Mice. Overlapping Roles of Adh1 and Adh4 in Ethanol Clearance and Metabolism of Retinol to Retinoic Acid. J. Biol. Chem. 1999, 274, 16796–16801.
- Molotkov, A.; Fan, X.; Duester, G. Excessive Vitamin A Toxicity in Mice Genetically Deficient in Either Alcohol Dehydrogenase Adh1 or Adh3. Eur. J. Biochem. 2002, 269, 2607–2612.
- Parker, R.O.; Crouch, R.K. Retinol Dehydrogenases (RDHs) in the Visual Cycle. Exp. Eye Res. 2010, 91, 788–792.
- Niederreither, K.; McCaffery, P.; Dräger, U.C.; Chambon, P.; Dollé, P. Restricted Expression and Retinoic Acid-Induced Downregulation of the Retinaldehyde Dehydrogenase Type 2 (RALDH-2) Gene during Mouse Development. Mech. Dev. 1997, 62, 67–78.
- Rochette-Egly, C. Retinoic Acid Signaling and Mouse Embryonic Stem Cell Differentiation: Cross Talk between Genomic and Non-Genomic Effects of RA. Biochim. Biophys. Acta 2015, 1851, 66–75.
- Kam, R.K.T.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic Acid Synthesis and Functions in Early Embryonic Development. Cell Biosci. 2012, 2, 11.
- Balmer, J.E.; Blomhoff, R. Gene Expression Regulation by Retinoic Acid. J. Lipid. Res. 2002, 43, 1773–1808.
- Brtko, J.; Dvorak, Z. Natural and Synthetic Retinoid X Receptor Ligands and Their Role in Selected Nuclear Receptor Action. Biochimie 2020, 179, 157–168.
- Thacher, S.; Vasudevan, J.; Chandraratna, R. Therapeutic Applications for Ligands of Retinoid Receptors. CPD 2000, 6, 25–58.
- de Almeida, N.R.; Conda-Sheridan, M. A Review of the Molecular Design and Biological Activities of RXR Agonists. Med. Res. Rev. 2019, 39, 1372–1397.
- Asson-Batres, M.A.; Rochette-Egly, C. (Eds.) The Biochemistry of Retinoic Acid Receptors I: Structure, Activation, and Function at the Molecular Level; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; Volume 70, ISBN 978-94-017-9049-9.
- Roy, B.; Taneja, R.; Chambon, P. Synergistic Activation of Retinoic Acid (RA)-Responsive Genes and Induction of Embryonal Carcinoma Cell Differentiation by an RA Receptor Alpha (RAR Alpha)-, RAR Beta-, or RAR Gamma-Selective Ligand in Combination with a Retinoid X Receptor-Specific Ligand. Mol. Cell Biol. 1995, 15, 6481–6487.
- Taneja, R.; Roy, B.; Plassat, J.L.; Zusi, C.F.; Ostrowski, J.; Reczek, P.R.; Chambon, P. Cell-Type and Promoter-Context Dependent Retinoic Acid Receptor (RAR) Redundancies for RAR Beta 2 and Hoxa-1 Activation in F9 and P19 Cells Can Be Artefactually Generated by Gene Knockouts. Proc. Natl. Acad. Sci. USA 1996, 93, 6197–6202.
- Chiba, H.; Clifford, J.; Metzger, D.; Chambon, P. Specific and Redundant Functions of Retinoid X Receptor/Retinoic Acid Receptor Heterodimers in Differentiation, Proliferation, and Apoptosis of F9 Embryonal Carcinoma Cells. J. Cell Biol. 1997, 139, 735–747.
- Mendoza-Parra, M.A.; Walia, M.; Sankar, M.; Gronemeyer, H. Dissecting the Retinoid-Induced Differentiation of F9 Embryonal Stem Cells by Integrative Genomics. Mol. Syst. Biol. 2011, 7, 538.
- Mendoza-Parra, M.-A.; Malysheva, V.; Mohamed Saleem, M.A.; Lieb, M.; Godel, A.; Gronemeyer, H. Reconstructed Cell Fate-Regulatory Programs in Stem Cells Reveal Hierarchies and Key Factors of Neurogenesis. Genome Res. 2016, 26, 1505–1519.
- Koshy, A.; Mathieux, E.; Stüder, F.; Bramoulle, A.; Lieb, M.; Colombo, B.M.; Gronemeyer, H.; Mendoza-Parra, M.A. Synergistic Activation of RARβ and RARγ Nuclear Receptors Restores Cell Specialization during Stem Cell Differentiation by Hijacking RARα-Controlled Programs. Life Sci. Alliance 2023, 6, e202201627.
- Dollé, P.; Izpisúa-Belmonte, J.C.; Falkenstein, H.; Renucci, A.; Duboule, D. Coordinate Expression of the Murine Hox-5 Complex Homoeobox-Containing Genes during Limb Pattern Formation. Nature 1989, 342, 767–772.
- Ruberte, E.; Dolle, P.; Krust, A.; Zelent, A.; Morriss-Kay, G.; Chambon, P. Specific Spatial and Temporal Distribution of Retinoic Acid Receptor Gamma Transcripts during Mouse Embryogenesis. Development 1990, 108, 213–222.
- Ruberte, E.; Dolle, P.; Chambon, P.; Morriss-Kay, G. Retinoic Acid Receptors and Cellular Retinoid Binding Proteins. II. Their Differential Pattern of Transcription during Early Morphogenesis in Mouse Embryos. Development 1991, 111, 45–60.
- Hale, L.A.; Tallafuss, A.; Yan, Y.-L.; Dudley, L.; Eisen, J.S.; Postlethwait, J.H. Characterization of the Retinoic Acid Receptor Genes Raraa, Rarab and Rarg during Zebrafish Development. Gene Expr. Patterns 2006, 6, 546–555.
- Tallafuss, A.; Hale, L.A.; Yan, Y.-L.; Dudley, L.; Eisen, J.S.; Postlethwait, J.H. Characterization of Retinoid-X Receptor Genes Rxra, Rxrba, Rxrbb and Rxrg during Zebrafish Development. Gene Expr. Patterns 2006, 6, 556–565.
- Waxman, J.S.; Yelon, D. Comparison of the Expression Patterns of Newly Identified Zebrafish Retinoic Acid and Retinoid X Receptors. Dev. Dyn. 2007, 236, 587–595.
- Blumberg, B.; Mangelsdorf, D.J.; Dyck, J.A.; Bittner, D.A.; Evans, R.M.; De Robertis, E.M. Multiple Retinoid-Responsive Receptors in a Single Cell: Families of Retinoid “X” Receptors and Retinoic Acid Receptors in the Xenopus Egg. Proc. Natl. Acad. Sci. USA 1992, 89, 2321–2325.
- Ellinger-Ziegelbauer, H.; Dreyer, C. A Retinoic Acid Receptor Expressed in the Early Development of Xenopus Laevis. Genes Dev. 1991, 5, 94–104.
- Koide, T.; Downes, M.; Chandraratna, R.A.; Blumberg, B.; Umesono, K. Active Repression of RAR Signaling Is Required for Head Formation. Genes Dev. 2001, 15, 2111–2121.
- Shiotsugu, J.; Katsuyama, Y.; Arima, K.; Baxter, A.; Koide, T.; Song, J.; Chandraratna, R.A.S.; Blumberg, B. Multiple Points of Interaction between Retinoic Acid and FGF Signaling during Embryonic Axis Formation. Development 2004, 131, 2653–2667.
- Biga, L.M.; Bronson, S.; Dawson, S.; Harwell, A.; Hopkins, R.; Kaufmann, J.; LeMaster, M.; Matern, P.; Morrison-Graham, K.; Oja, K.; et al. Anatomy & Physiology; OpenStax/Oregon State University: Corvallis, OR, USA, 2019.
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic Retinoic Acid Synthesis Is Essential for Early Mouse Post-Implantation Development. Nat. Genet. 1999, 21, 444–448.
- Mollard, R.; Viville, S.; Ward, S.J.; Décimo, D.; Chambon, P.; Dollé, P. Tissue-Specific Expression of Retinoic Acid Receptor Isoform Transcripts in the Mouse Embryo. Mech. Dev. 2000, 94, 223–232.
- Ruberte, E.; Friederich, V.; Chambon, P.; Morriss-Kay, G. Retinoic Acid Receptors and Cellular Retinoid Binding Proteins III. Their Differential Transcript Distribution during Mouse Nervous System Development. Development 1993, 118, 267–282.
- Dollé, P. Developmental Expression of Retinoic Acid Receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006.
- Allen Institute for Brain Science. Allen Mouse Brain Atlas . 2013. Available online: http://mouse.brain-map.org/ (accessed on 4 January 2022).
- Liu, Y.; Yang, M.; Deng, Y.; Su, G.; Enninful, A.; Guo, C.C.; Tebaldi, T.; Zhang, D.; Kim, D.; Bai, Z.; et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell 2020, 183, 1665–1681.e18.
- Moehlin, J.; Mollet, B.; Colombo, B.M.; Mendoza-Parra, M.A. Inferring Biologically Relevant Molecular Tissue Substructures by Agglomerative Clustering of Digitized Spatial Transcriptomes with Multilayer. Cell Syst. 2021, 12, 694–705.e3.
- Protocol for Using MULTILAYER to Reveal Molecular Tissue Substructures from Digitized Spatial Transcriptomes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34585159/ (accessed on 4 January 2022).
- Deng, Y.; Bartosovic, M.; Kukanja, P.; Zhang, D.; Liu, Y.; Su, G.; Enninful, A.; Bai, Z.; Castelo-Branco, G.; Fan, R. Spatial-CUT&Tag: Spatially Resolved Chromatin Modification Profiling at the Cellular Level. Science 2022, 375, 681–686.
- Barnett, J.; Sotudeh, N.; Rao, P.; Silverman, J.; Jafar, T.; Wang, L. AtlasXplore: A Web Platform for Visualizing and Sharing Spatial Epigenome Data. Bioinformatics 2023, 39, btad447.
- Zhang, T.; Cooper, S.; Brockdorff, N. The Interplay of Histone Modifications—Writers That Read. EMBO Rep. 2015, 16, 1467–1481.
- Uehara, M.; Yashiro, K.; Mamiya, S.; Nishino, J.; Chambon, P.; Dolle, P.; Sakai, Y. CYP26A1 and CYP26C1 Cooperatively Regulate Anterior-Posterior Patterning of the Developing Brain and the Production of Migratory Cranial Neural Crest Cells in the Mouse. Dev. Biol. 2007, 302, 399–411.
- Hernandez, R.E.; Putzke, A.P.; Myers, J.P.; Margaretha, L.; Moens, C.B. Cyp26 Enzymes Generate the Retinoic Acid Response Pattern Necessary for Hindbrain Development. Development 2007, 134, 177–187.
- Ribes, V.; Otto, D.M.E.; Dickmann, L.; Schmidt, K.; Schuhbaur, B.; Henderson, C.; Blomhoff, R.; Wolf, C.R.; Tickle, C.; Dollé, P. Rescue of Cytochrome P450 Oxidoreductase (Por) Mouse Mutants Reveals Functions in Vasculogenesis, Brain and Limb Patterning Linked to Retinoic Acid Homeostasis. Dev. Biol. 2007, 303, 66–81.
- Novitch, B.G.; Wichterle, H.; Jessell, T.M.; Sockanathan, S. A Requirement for Retinoic Acid-Mediated Transcriptional Activation in Ventral Neural Patterning and Motor Neuron Specification. Neuron 2003, 40, 81–95.
- Wilson, L.; Maden, M. The Mechanisms of Dorsoventral Patterning in the Vertebrate Neural Tube. Dev. Biol. 2005, 282, 1–13.
- Diez del Corral, R.; Storey, K.G. Opposing FGF and Retinoid Pathways: A Signalling Switch That Controls Differentiation and Patterning Onset in the Extending Vertebrate Body Axis. Bioessays 2004, 26, 857–869.
