Allergy is a type 2 immune reaction triggered by antigens known as allergens, including food and environmental substances such as peanuts, plant pollen, fungal spores, and the feces and debris of mites and insects. Macrophages are myeloid immune cells with phagocytic abilities that process exogenous and endogenous antigens. Upon activation, they can produce effector molecules such as cytokines as well as anti-inflammatory molecules. The dysregulation of macrophage function can lead to excessive type 1 inflammation as well as type 2 inflammation, which includes allergic reactions. Thus, it is important to better understand how macrophages are regulated in the pathogenesis of allergies. Emerging evidence highlights the role of noncoding RNAs (ncRNAs) in macrophage polarization, which in turn can modify the pathogenesis of various immune-mediated diseases, including allergies.
- macrophage
- macrophage polarization
- allergy
- non-coding RNA
1. Introduction
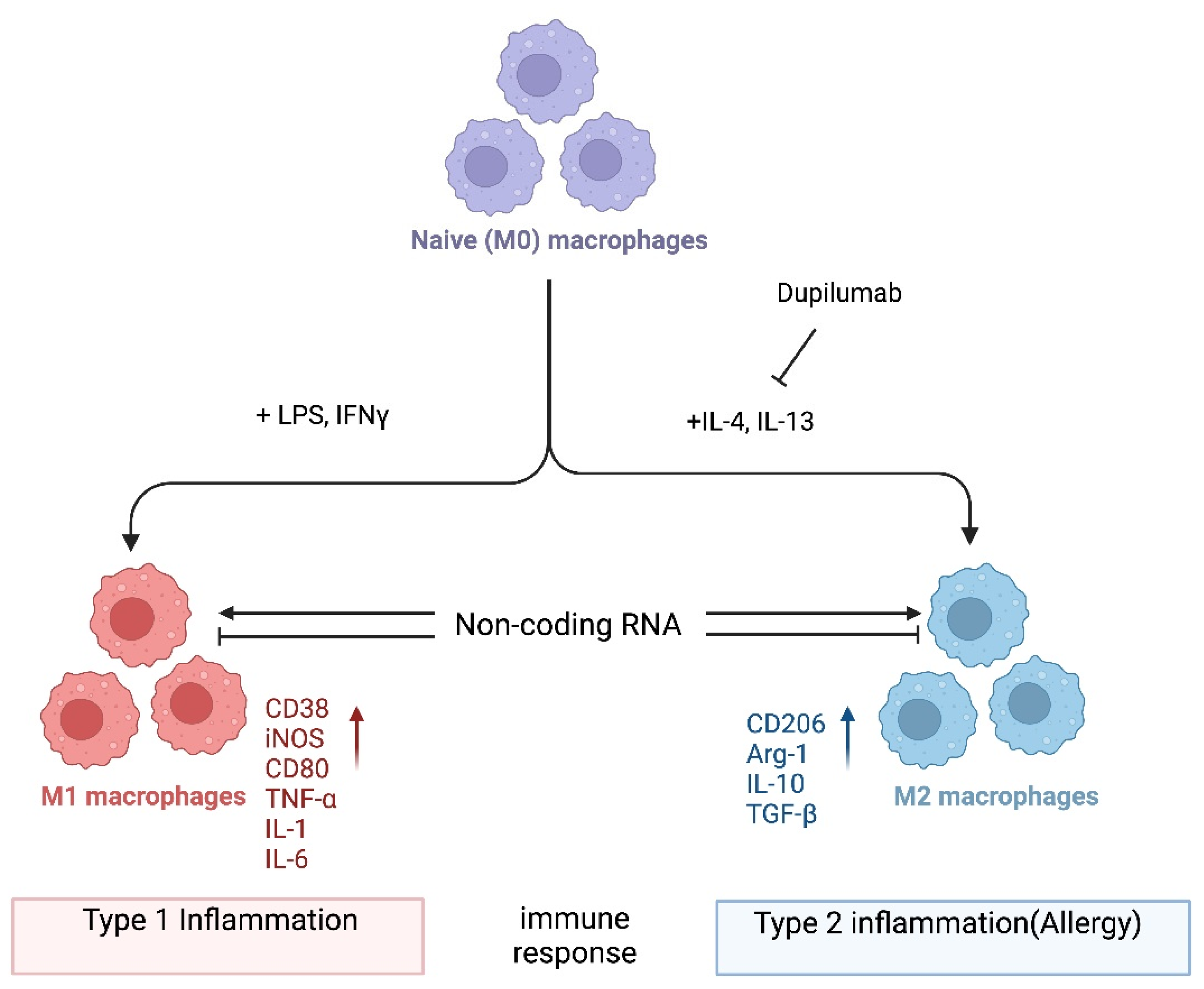
2. miRNA-Mediated Regulation of Macrophage Polarization
3. lncRNA-Mediated Regulation of Macrophage Polarization
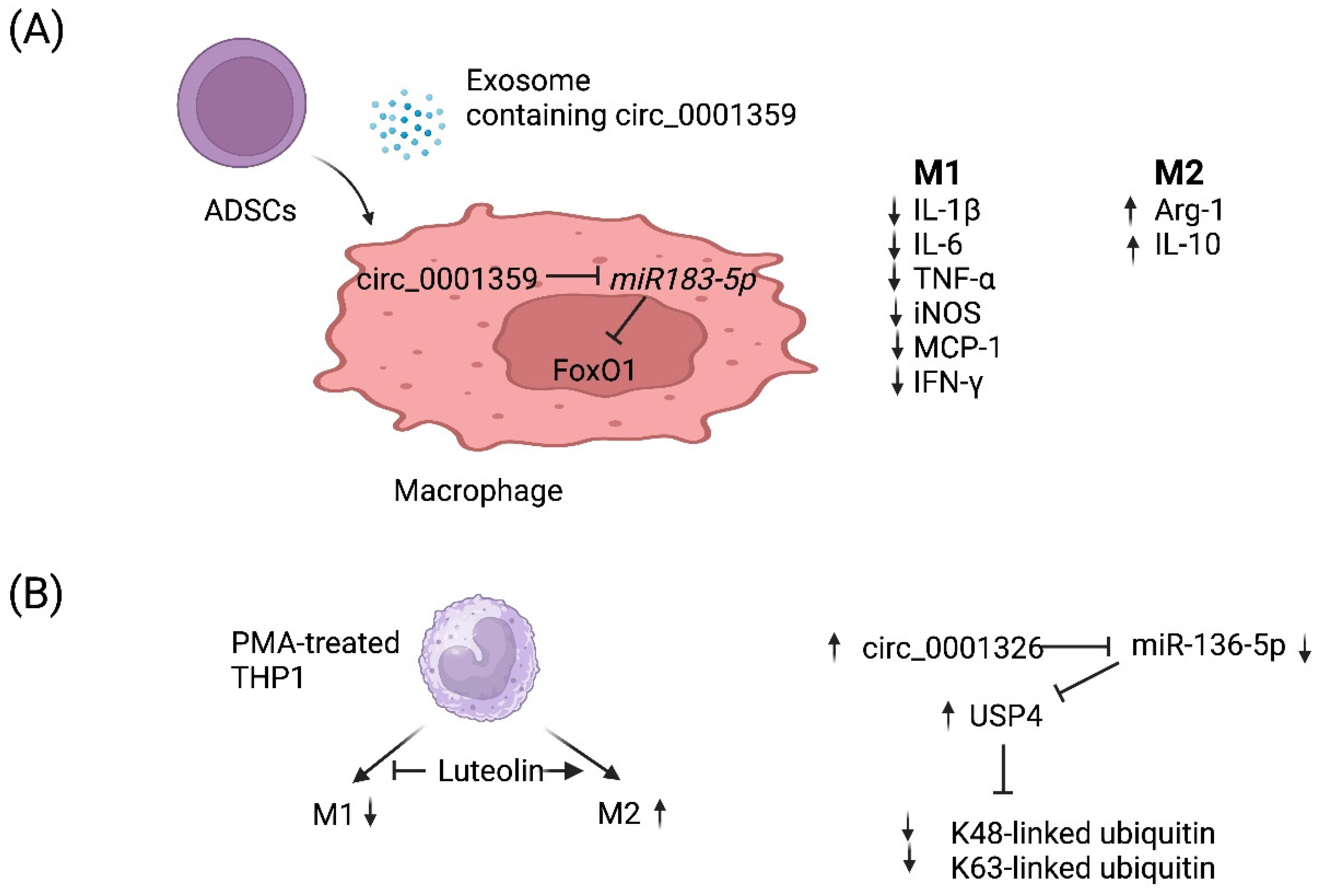
4. circRNA-Mediated Regulation of Macrophage Polarization
This entry is adapted from the peer-reviewed paper 10.3390/ncrna9060075
References
- Lee, T.H. Allergy: The unmet need. Clin. Med. 2003, 3, 303–305.
- Pulendran, B.; Artis, D. New Paradigms in Type 2 Immunity. Science 2012, 337, 431–435.
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F.; Blaiss, M.S. WAO White Book on Allergy: Update 2013 Executive Summary; World Allergy Organization: Milwaukee, WI, USA, 2013.
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. Allergic diseases and asthma: A major global health concern. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 39–41.
- Pawankar, R.; Mori, S.; Ozu, C.; Kimura, S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy 2011, 1, 157–167.
- King, T.P.; Norman, P.S. Isolation studies of allergens from regweed pollen. Biochemistry 1962, 1, 709–720.
- Sudharson, S.; Kalic, T.; Hafner, C.; Breiteneder, H. Newly defined allergens in the WHO/IUIS Allergen Nomenclature Database during 01/2019-03/2021. Allergy 2021, 76, 3359–3373.
- Thompson, C.P.; Silvers, S.; Shapiro, M.A. Intralymphatic immunotherapy for mountain cedar pollinosis: A randomized, double-blind, placebo-controlled trial. Ann. Allergy Asthma Immunol. 2020, 125, 311–318.e2.
- James, C.; Bernstein, D.I. Allergen immunotherapy: An updated review of safety. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 55–59.
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190.
- Girodet, P.O.; Nguyen, D.; Mancini, J.D.; Hundal, M.; Zhou, X.; Israel, E.; Cernadas, M. Alternative Macrophage Activation Is Increased in Asthma. Am. J. Respir. Cell Mol. Biol. 2016, 55, 467–475.
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58.
- Balhara, J.; Gounni, A.S. The alveolar macrophages in asthma: A double-edged sword. Mucosal Immunol. 2012, 5, 605–609.
- van der Veen, T.A.; de Groot, L.E.; Melgert, B.N. The different faces of the macrophage in asthma. Curr. Opin. Pulm. Med. 2020, 26, 62–68.
- Hou, Y.; Wei, D.; Zhang, Z.; Guo, H.; Li, S.; Zhang, J.; Zhang, P.; Zhang, L.; Zhao, Y. FABP5 controls macrophage alternative activation and allergic asthma by selectively programming long-chain unsaturated fatty acid metabolism. Cell Rep. 2022, 41, 111668.
- Mackaness, G.B. Cellular resistance to infection. J. Exp. Med. 1962, 116, 381–406.
- Robbe, P.; Draijer, C.; Borg, T.R.; Luinge, M.; Timens, W.; Wouters, I.M.; Melgert, B.N.; Hylkema, M.N. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L358–L367.
- Draijer, C.; Robbe, P.; Boorsma, C.E.; Hylkema, M.N.; Melgert, B.N. Dual role of YM1+ M2 macrophages in allergic lung inflammation. Sci. Rep. 2018, 8, 5105.
- Zhu, L.; Zhao, Q.; Yang, T.; Ding, W.; Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2015, 34, 82–100.
- Bertani, F.R.; Mozetic, P.; Fioramonti, M.; Iuliani, M.; Ribelli, G.; Pantano, F.; Santini, D.; Tonini, G.; Trombetta, M.; Businaro, L.; et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci. Rep. 2017, 7, 8965.
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969.
- Islam, Z.; Inui, T.; Ishibashi, O. Gpr137b is an orphan G-protein-coupled receptor associated with M2 macrophage polarization. Biochem. Biophys. Res. Commun. 2019, 509, 657–663.
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983, 158, 670–689.
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 6–13.
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20.
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795.
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995.
- Li, J.; Kim, S.Y.; Lainez, N.M.; Coss, D.; Nair, M.G. Macrophage-Regulatory T Cell Interactions Promote Type 2 Immune Homeostasis Through Resistin-Like Molecule α. Front. Immunol. 2021, 12, 710406.
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614.
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389.
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204.
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92.
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16, 2019-0027.
- Tian, C.; Gao, J.; Yang, L.; Yuan, X. Non-coding RNA regulation of macrophage function in asthma. Cell. Signal. 2023, 112, 110926.
- Feketea, G.; Bocsan, C.I.; Popescu, C.; Gaman, M.; Stanciu, L.A.; Zdrenghea, M.T. A Review of Macrophage MicroRNAs’ Role in Human Asthma. Cells 2019, 8, 420.
- Peschansky, V.J.; Wahlestedt, C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 2014, 9, 3–12.
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 159.
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447.
- Goodarzi, V.; Nouri, S.; Nassaj, Z.S.; Bighash, M.; Abbasian, S.; Hagh, R.A. Long non coding RNAs reveal important pathways in childhood asthma: A future perspective. Histochem. J. 2023, 54, 257–269.
- Squadrito, M.L.; Etzrodt, M.; De Palma, M.; Pittet, M.J. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013, 34, 350–359.
- Martinez-Pomares, L. The mannose receptor. J. Leukoc Biol. 2012, 92, 1177–1186.
- Squadrito, M.L.; Pucci, F.; Magri, L.; Moi, D.; Gilfillan, G.D.; Ranghetti, A.; Casazza, A.; Mazzone, M.; Lyle, R.; Naldini, L.; et al. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep. 2012, 1, 141–154.
- Zhou, Y.; Do, D.C.; Ishmael, F.T.; Squadrito, M.L.; Tang, H.M.; Tang, H.L.; Hsu, M.H.; Qiu, L.; Li, C.; Zhang, Y.; et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J. Allergy Clin. Immunol. 2018, 141, 350–364.e8.
- Do, D.C.; Mu, J.; Ke, X.; Sachdeva, K.; Qin, Z.; Wan, M.; Ishmael, F.T.; Gao, P. miR-511-3p protects against cockroach allergen-induced lung inflammation by antagonizing CCL2. J. Clin. Investig. 2019, 4, e126832.
- Heinsbroek, S.E.; Squadrito, M.L.; Schilderink, R.; Hilbers, F.W.; Verseijden, C.; Hofmann, M.; Helmke, A.; Boon, L.; Wildenberg, M.E.; Roelofs, J.J.; et al. miR-511-3p, embedded in the macrophage mannose receptor gene, contributes to intestinal inflammation. Mucosal Immunol. 2016, 9, 960–973.
- Chung, S.; Lee, Y.G.; Karpurapu, M.; Englert, J.A.; Ballinger, M.N.; Davis, I.C.; Park, G.Y.; Christman, J.W. Depletion of microRNA-451 in response to allergen exposure accentuates asthmatic inflammation by regulating Sirtuin2. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L921–L930.
- Veremeyko, T.; Siddiqui, S.; Sotnikov, I.; Yung, A.; Ponomarev, E.D. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS ONE 2013, 8, e81774.
- Shi, J.; Chen, M.; Ouyang, L.; Wang, Q.; Guo, Y.; Huang, L.; Jiang, S. miR-142-5p and miR-130a-3p regulate pulmonary macrophage polarization and asthma airway remodeling. Immunol. Cell Biol. 2020, 98, 715–725.
- Su, S.; Zhao, Q.; He, C.; Huang, D.; Liu, J.; Chen, F.; Chen, J.; Liao, J.Y.; Cui, X.; Zeng, Y.; et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015, 6, 8523.
- Paoletti, A.; Rohmer, J.; Ly, B.; Pascaud, J.; Rivière, E.; Seror, R.; Le Goff, B.; Nocturne, G.; Mariette, X. Monocyte/Macrophage Abnormalities Specific to Rheumatoid Arthritis Are Linked to miR-155 and Are Differentially Modulated by Different TNF Inhibitors. J. Immunol. 2019, 203, 1766–1775.
- Han, X.; Huang, S.; Xue, P.; Fu, J.; Liu, L.; Zhang, C.; Yang, L.; Xia, L.; Sun, L.; Huang, S.K.; et al. LncRNA PTPRE-AS1 modulates M2 macrophage activation and inflammatory diseases by epigenetic promotion of PTPRE. Sci. Adv. 2019, 5, eaax9230.
- Wen, S.; Li, F.; Tang, Y.; Dong, L.; He, Y.; Deng, Y.; Tao, Z. MIR222HG attenuates macrophage M2 polarization and allergic inflammation in allergic rhinitis by targeting the miR146a-5p/TRAF6/NF-κB axis. Front. Immunol. 2023, 14, 1168920.
- Pei, W.; Zhang, Y.; Li, X.; Luo, M.; Chen, T.; Zhang, M.; Zhong, M.; Lv, K. LncRNA AK085865 depletion ameliorates asthmatic airway inflammation by modulating macrophage polarization. Int. Immunopharmacol. 2020, 83, 106450.
- Zhang, Y.; Li, X.; Wang, C.; Zhang, M.; Yang, H.; Lv, K. lncRNA AK085865 Promotes Macrophage M2 Polarization in CVB3-Induced VM by Regulating ILF2-ILF3 Complex-Mediated miRNA-192 Biogenesis. Mol. Ther. Nucleic Acids 2020, 21, 441–451.
- Xia, L.; Wang, X.; Liu, L.; Fu, J.; Xiao, W.; Liang, Q.; Han, X.; Huang, S.; Sun, L.; Gao, Y.; et al. lnc-BAZ2B promotes M2 macrophage activation and inflammation in children with asthma through stabilizing BAZ2B pre-mRNA. J. Allergy Clin. Immunol. 2021, 147, 921–932.e9.
- Li, Q.; Lu, L.; Li, X.; Lu, S. Long non-coding RNA NKILA alleviates airway inflammation in asthmatic mice by promoting M2 macrophage polarization and inhibiting the NF-κB pathway. Biochem. Biophys. Res. Commun. 2021, 571, 46–52.
- Jiang, M.; Dai, J.; Yin, M.; Jiang, C.; Ren, M.; Tian, L. LncRNA MEG8 sponging miR-181a-5p contributes to M1 macrophage polarization by regulating SHP2 expression in Henoch-Schonlein purpura rats. Ann. Med. 2021, 53, 1576–1588.
- Zhu, X.; Sun, Y.; Yu, Q.; Wang, X.; Wang, Y.; Zhao, Y. Exosomal lncRNA GAS5 promotes M1 macrophage polarization in allergic rhinitis via restraining mTORC1/ULK1/ATG13-mediated autophagy and subsequently activating NF-κB signaling. Int. Immunopharmacol. 2023, 121, 110450.
- Yu, C.-Y.; Kuo, H.-C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29.
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338.
- Zang, J.; Lu, D.; Xu, A. The interaction of circRNAs and RNA binding proteins: An important part of circRNA maintenance and function. J. Neurosci. Res. 2020, 98, 87–97.
- Shang, Y.; Sun, Y.; Xu, J.; Ge, X.; Hu, Z.; Xiao, J.; Ning, Y.; Dong, Y.; Bai, C. Exosomes from mmu_circ_0001359-Modified ADSCs Attenuate Airway Remodeling by Enhancing FoxO1 Signaling-Mediated M2-like Macrophage Activation. Mol. Ther. Nucleic Acids 2020, 19, 951–960.
- Gong, B.; Zheng, Y.; Li, J.; Lei, H.; Liu, K.; Tang, J.; Peng, Y. Luteolin activates M2 macrophages and suppresses M1 macrophages by upregulation of hsa_circ_0001326 in THP-1 derived macrophages. Bioengineered 2022, 13, 5079–5090.
