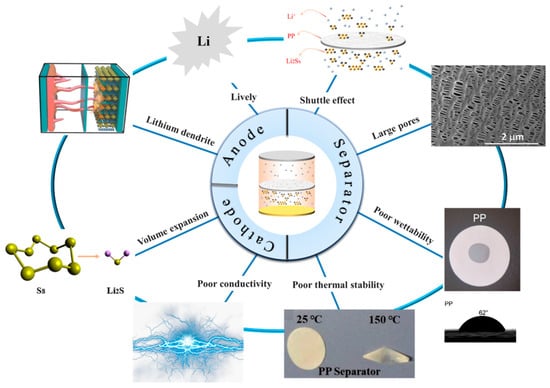
Lithium–sulfur (Li-S) batteries are considered one of the most promising energy storage systems due to their high theoretical capacity, high theoretical capacity density, and low cost. However, challenges such as poor conductivity of sulfur (S) elements in active materials, the “shuttle effect” caused by lithium polysulfide, and the growth of lithium dendrites impede the commercial development of Li-S batteries. As a crucial component of the battery, the separator plays a vital role in mitigating the shuttle effect caused by polysulfide. Traditional polypropylene, polyethylene, and polyimide separators are constrained by their inherent limitations, rendering them unsuitable for direct application in lithium–sulfur batteries. Therefore, there is an urgent need for the development of novel separators.
- lithium–sulfur batteries
- shuttle effect
- separator
- separator modification
1. Introduction
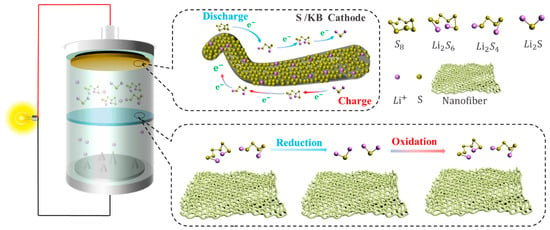
2. The Working Principle and Problems of Li-S
2.1. Working Mechanism of Li-S
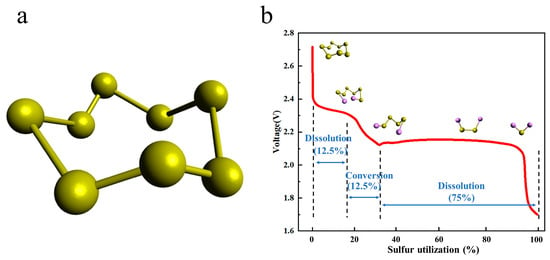
2.2. The Existing Problems of Li-S
2.2.1. Cathode
2.2.2. Separator
2.2.3. Anode
2.2.4. Electrolyte
2.2.5. Binders
2.2.6. Current Collector
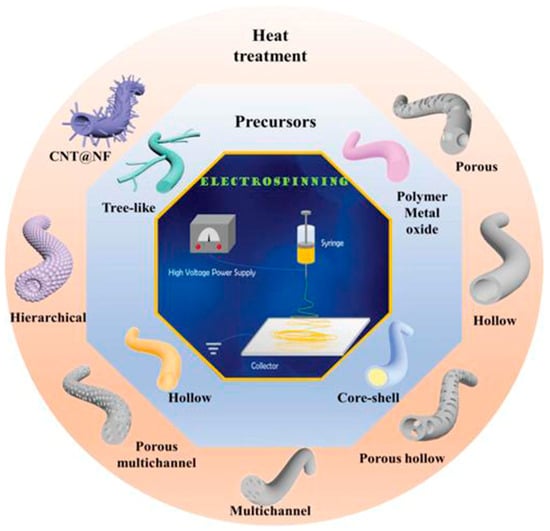
3. Methods
3.1. Electrospinning
3.2. Vacuum Filtration
3.3. Wet Spinning
3.4. Coating Method
3.5. In Situ Growth Method
3.6. Atomic Layer Deposition (ALD)
4. Application of Separators in Lithium–Sulfur Batteries
4.1. Electrospinning
4.1.1. Separator
4.1.2. Interlayer
4.2. Vacuum Filtration
4.2.1. Separator
4.2.2. Interlayer
4.3. Wet Spinning
4.4. Coating Method
4.4.1. Separator
4.4.2. Interlayer
4.5. In Situ Growth Method
4.5.1. Separator
4.5.2. Interlayer
4.6. Atomic Layer Deposition
4.6.1. Separator
4.6.2. Interlayer
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/polym15193955
References
- Kang, N.; Lin, Y.X.; Yang, L.; Lu, D.P.; Xiao, J.; Qi, Y.; Cai, M. Cathode porosity is a missing key parameter to optimize lithium-sulfur battery energy density. Nat. Commun. 2019, 10, 4597.
- Liu, M.; Deng, N.P.; Ju, J.G.; Fan, L.L.; Wang, L.Y.; Li, Z.J.; Zhao, H.J.; Yang, G.; Kang, W.M.; Yan, J.; et al. A Review: Electrospun Nanofiber Materials for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1905467.
- Din, M.M.U.; Murugan, R. Metal Coated Polypropylene Separator with Enhanced Surface Wettability for High Capacity Lithium Metal Batteries. Sci. Rep. 2019, 9, 16795.
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational Design of Nanostructured Functional Interlayer/Separator for Advanced Li-S Batteries. Adv. Funct. Mater. 2018, 28, 1707411.
- Cuisinier, M.; Hart, C.; Balasubramanian, M.; Garsuch, A.; Nazar, L.F. Radical or Not Radical: Revisiting Lithium-Sulfur Electrochemistry in Nonaqueous Electrolytes. Adv. Energy Mater. 2015, 5, 1401801.
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527.
- Song, Y.Z.; Cai, W.L.; Kong, L.; Cai, J.S.; Zhang, Q.; Sun, J.Y. Rationalizing Electrocatalysis of Li-S Chemistry by Mediator Design: Progress and Prospects. Adv. Energy Mater. 2020, 10, 1901075.
- Ren, W.C.; Ma, W.; Zhang, S.F.; Tang, B.T. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732.
- Liu, H.; Lai, W.-H.; Yang, H.-L.; Zhu, Y.-F.; Lei, Y.-J.; Zhao, L.; Peng, J.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K. Efficient separators with fast Li-ion transfer and high polysulfide entrapment for superior lithium-sulfur batteries. Chem. Eng. J. 2021, 408, 127348.
- Phung, J.; Zhang, X.Z.; Deng, W.J.; Li, G. An overview of MOF-based separators for lithium-sulfur batteries. Sustain. Mater. Technol. 2022, 31, e00374.
- Zhang, Y.S.; Zhang, X.L.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G.S. Lithium-Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 2103879.
- Zhang, Y.G.; Yang, S.; Zhou, S.Y.; Zhang, L.B.; Gu, B.B.; Dong, Y.Y.; Kong, S.Z.; Cai, D.; Fang, G.Y.; Nie, H.G.; et al. Oxygen doping in antimony sulfide nanosheets to facilitate catalytic conversion of polysulfides for lithium-sulfur batteries. Chem. Commun. 2021, 57, 3255–3258.
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134.
- Cuisinier, M.; Cabelguen, P.E.; Evers, S.; He, G.; Kolbeck, M.; Garsuch, A.; Bolin, T.; Balasubramanian, M.; Nazar, L.F. Sulfur Speciation in Li-S Batteries Determined by Operando X-ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3227–3232.
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Analysis of Polysulfide Dissolved in Electrolyte in Discharge-Charge Process of Li-S Battery. J. Electrochem. Soc. 2012, 159, A421–A425.
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494.
- Zhao, E.Y.; Nie, K.H.; Yu, X.Q.; Hu, Y.S.; Wang, F.W.; Xiao, J.; Li, H.; Huang, X.J. Advanced Characterization Techniques in Promoting Mechanism Understanding for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707543.
- Sun, J.; Sun, Y.M.; Pasta, M.; Zhou, G.M.; Li, Y.Z.; Liu, W.; Xiong, F.; Cui, Y. Entrapment of Polysulfides by a Black-Phosphorus-Modified Separator for Lithium-Sulfur Batteries. Adv. Mater. 2016, 28, 9797–9803.
- Yao, S.S.; Cui, J.; Huang, J.Q.; Lu, Z.H.; Deng, Y.; Chong, W.G.; Wu, J.X.; Ihsan Ul Haq, M.; Ciucci, F.; Kim, J.K. Novel 2D Sb2S3 Nanosheet/CNT Coupling Layer for Exceptional Polysulfide Recycling Performance. Adv. Energy Mater. 2018, 8, 1800710.
- Park, J.; Kim, E.T.; Kim, C.; Pyun, J.; Jang, H.S.; Shin, J.; Choi, J.W.; Char, K.; Sung, Y.E. The Importance of Confined Sulfur Nanodomains and Adjoining Electron Conductive Pathways in Subreaction Regimes of Li-S Batteries. Adv. Energy Mater. 2017, 7, 1700074.
- Wu, J.Y.; Zeng, H.X.; Li, X.W.; Pei, H.J.; Xue, Z.G.; Ye, Y.S.; Xie, X.L. Dual-Functional Interlayer Based on Radially Oriented Ultrathin MoS2 Nanosheets for High-Performance Lithium Sulfur-Batteries. Acs Appl. Energy Mater. 2019, 2, 1702–1711.
- Zhao, M.; Li, B.-Q.; Chen, X.; Xie, J.; Yuan, H.; Huang, J.-Q. Redox Comediation with Organopolysulfides in Working Lithium-Sulfur Batteries. Chem 2020, 6, 3297–3311.
- Zhao, M.; Peng, Y.-Q.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q. Regulation of carbon distribution to construct high-sulfur-content cathode in lithium–sulfur batteries. J. Energy Chem. 2021, 56, 203–208.
- Sadd, M.; De Angelis, S.; Colding-Jorgensen, S.; Blanchard, D.; Johnsen, R.E.; Sanna, S.; Borisova, E.; Matic, A.; Bowen, J.R. Visualization of Dissolution-Precipitation Processes in Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103126.
- Ren, Y.X.; Zhao, T.S.; Liu, M.; Tan, P.; Zeng, Y.K. Modeling of lithium-sulfur batteries incorporating the effect of Li2S precipitation. J. Power Sources 2016, 336, 115–125.
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908.
- Guo, J.; Yang, Z.; Yu, Y.; Abruna, H.D.; Archer, L.A. Lithium-sulfur battery cathode enabled by lithium-nitrile interaction. J. Am. Chem. Soc. 2013, 135, 763–767.
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647.
- Barai, P.; Mistry, A.; Mukherjee, P.P. Poromechanical effect in the lithium–sulfur battery cathode. Extrem. Mech. Lett. 2016, 9, 359–370.
- Yan, J.H.; Liu, X.B.; Li, B.Y. Capacity Fade Analysis of Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1600101.
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, N.A.; Liang, B.; Iqbal, A.; Wang, J.X.; Sin, H.S.; Li, L.S.; Tang, Z.Y. MoS2/Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1606817.
- Zhai, P.; Liu, K.; Wang, Z.; Shi, L.; Yuan, S. Multifunctional separators for high-performance lithium-ion batteries. J. Power Sources 2021, 499, 229973.
- Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; He, W.; et al. Inhibiting Polysulfide Shuttling with a Graphene Composite Separator for Highly Robust Lithium-Sulfur Batteries. Joule 2018, 2, 2091–2104.
- Gao, Z.; Xue, Z.; Miao, Y.; Chen, B.; Xu, J.; Shi, H.; Tang, T.; Zhao, X. TiO2@Porous carbon nanotubes modified separator as polysulfide barrier for lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164249.
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.-G. Anodes for Rechargeable Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1402273.
- Cheng, X.B.; Huang, J.Q.; Zhang, Q. Review-Li Metal Anode in Working Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A6058–A6072.
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Characterization of the solid electrolyte interphase on lithium anode for preventing the shuttle mechanism in lithium–sulfur batteries. J. Power Sources 2014, 246, 840–845.
- Rong, G.L.; Zhang, X.Y.; Zhao, W.; Qiu, Y.C.; Liu, M.N.; Ye, F.M.; Xu, Y.; Chen, J.F.; Hou, Y.; Li, W.F.; et al. Liquid-Phase Electrochemical Scanning Electron Microscopy for In Situ Investigation of Lithium Dendrite Growth and Dissolution. Adv. Mater. 2017, 29, 1606187.
- Wang, J.; Yi, S.; Liu, J.; Sun, S.; Liu, Y.; Yang, D.; Xi, K.; Gao, G.; Abdelkader, A.; Yan, W.; et al. Suppressing the Shuttle Effect and Dendrite Growth in Lithium-Sulfur Batteries. ACS Nano 2020, 14, 9819–9831.
- Rosso, M.; Brissot, C.; Teyssot, A.; Dolle, M.; Sannier, L.; Tarascon, J.M.; Bouchetc, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340.
- Fan, L.; Chen, S.H.; Zhu, J.Y.; Ma, R.F.; Li, S.P.; Podila, R.; Rao, A.M.; Yang, G.Z.; Wang, C.X.; Liu, Q.; et al. Simultaneous Suppression of the Dendrite Formation and Shuttle Effect in a Lithium-Sulfur Battery by Bilateral Solid Electrolyte Interface. Adv. Sci. 2018, 5, 1700934.
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Wang, H.; Wang, X.; Rao, G.; Wang, X.; Chen, B.; Xiong, J. Graphene quantum dots as the nucleation sites and interfacial regulator to suppress lithium dendrites for high-loading lithium-sulfur battery. Nano Energy 2020, 68, 104373.
- Wang, L.; Liu, J.; Yuan, S.; Wang, Y.; Xia, Y. To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 2016, 9, 224–231.
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162.
- Li, Y.; Wang, X.; Liang, J.; Wu, K.; Xu, L.; Wang, J. Design of a high performance zeolite/polyimide composite separator for lithium-ion batteries. Polymers 2020, 12, 764.
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262.
- Zhao, M.; Wang, J.; Chong, C.B.; Yu, X.W.; Wanga, L.L.; Shi, Z.Q. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120.
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391.
- Gao, W.L.; Kono, J. Science and applications of wafer-scale crystalline carbon nanotube films prepared through controlled vacuum filtration. R. Soc. Open Sci. 2019, 6, 181605.
- Kritzer, P. Nonwoven support material for improved separators in Li–polymer batteries. J. Power Sources 2006, 161, 1335–1340.
- Zhu, J.; Ge, Y.; Kim, D.; Lu, Y.; Chen, C.; Jiang, M.; Zhang, X. A novel separator coated by carbon for achieving exceptional high performance lithium-sulfur batteries. Nano Energy 2016, 20, 176–184.
- Shao, H.; Wang, W.; Zhang, H.; Wang, A.; Chen, X.; Huang, Y. Nano-TiO2 decorated carbon coating on the separator to physically and chemically suppress the shuttle effect for lithium-sulfur battery. J. Power Sources 2018, 378, 537–545.
- Yang, L.W.; Wang, Y.; Li, Q.; Li, Y.; Chen, Y.X.; Liu, Y.X.; Wu, Z.G.; Wang, G.K.; Zhong, B.H.; Song, Y.; et al. Inhibition of the shuttle effect of lithium-sulfur batteries via a tannic acid-metal one-step in situ chemical film-forming modified separator. Nanoscale 2021, 13, 5058–5068.
- Lin, Q.Y.; Ding, B.; Chen, S.; Li, P.; Li, Z.W.; Shi, Y.Y.; Dou, H.; Zhang, X.G. Atomic Layer Deposition of Single Atomic Cobalt as a Catalytic Interlayer for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 11206–11212.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347.
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309.
- Hao, J.; Lei, G.; Li, Z.; Wu, L.; Xiao, Q.; Wang, L. A novel polyethylene terephthalate nonwoven separator based on electrospinning technique for lithium ion battery. J. Membr. Sci. 2013, 428, 11–16.
- Zhang, L.Y.; Batchelor, W.; Varanasi, S.; Tsuzuki, T.; Wang, X.G. Effect of cellulose nanofiber dimensions on sheet forming through filtration. Cellulose 2012, 19, 561–574.
- Cho, T.-H.; Tanaka, M.; Ohnishi, H.; Kondo, Y.; Yoshikazu, M.; Nakamura, T.; Sakai, T. Composite nonwoven separator for lithium-ion battery: Development and characterization. J. Power Sources 2010, 195, 4272–4277.
- Safavi, A.; Fathi, S.; Babaei, M.R.; Mansoori, Z.; Latifi, M. Experimental and numerical analysis of fiber characteristics effects on fiber dispersion for wet-laid nonwoven. Fibers Polym. 2009, 10, 231–236.
- Wang, X.R.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904.
- Yan, B.; Li, X.; Bai, Z.; Song, X.; Xiong, D.; Zhao, M.; Li, D.; Lu, S. A review of atomic layer deposition providing high performance lithium sulfur batteries. J. Power Sources 2017, 338, 34–48.
- Zhu, X.B.; Ouyang, Y.; Chen, J.W.; Zhu, X.G.; Luo, X.; Lai, F.L.; Zhang, H.; Miao, Y.E.; Liu, T.X. In situ extracted poly(acrylic acid) contributing to electrospun nanofiber separators with precisely tuned pore structures for ultra-stable lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 3253–3263.
- Guo, P.; Jiang, P.; Chen, W.; Qian, G.; He, D.; Lu, X. Bifunctional Al2O3/polyacrylonitrile membrane to suppress the growth of lithium dendrites and shuttling of polysulfides in lithium-sulfur batteries. Electrochim. Acta 2022, 428, 140955.
- Guo, Y.; Li, J.; Pitcheri, R.; Zhu, J.; Wen, P.; Qiu, Y. Electrospun Ti4O7/C conductive nanofibers as interlayer for lithium-sulfur batteries with ultra long cycle life and high-rate capability. Chem. Eng. J. 2019, 355, 390–398.
- Huangfu, Y.; Zheng, T.; Zhang, K.; She, X.; Xu, H.; Fang, Z.; Xie, K. Facile fabrication of permselective g-C3N4 separator for improved lithium-sulfur batteries. Electrochim. Acta 2018, 272, 60–67.
- Feng, P.; Hou, W.; Bai, Z.; Bai, Y.; Sun, K.; Wang, Z. Ultrathin two-dimensional bimetal NiCo-based MOF nanosheets as ultralight interlayer in lithium-sulfur batteries. Chin. Chem. Lett. 2023, 34, 107427.
- Meng, L.; Li, Y.; Lin, Q.X.; Long, J.; Wang, Y.; Hu, J. Nitrogen and Oxygen Dual Self-Doped Flexible PPTA Nanofiber Carbon Paper as an Effective Interlayer for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2021, 4, 8592–8603.
- Li, Y.; Meng, L.; Jin, L.; Yun, L.; Jian, H. A wet-laid carbon paper with 3D conductive structure as an interlayer for lithium-sulfur batteries. Mater. Res. Express 2019, 6, 125547.
- Chong, W.G.; Xiao, F.; Yao, S.S.; Cui, J.; Sadighi, Z.; Wu, J.X.; Ihsan-Ul-Haq, M.; Shao, M.H.; Kim, J.K. Nitrogen-doped graphene fiber webs for multi-battery energy storage. Nanoscale 2019, 11, 6334–6342.
- Suriyakumar, S.; Stephan, A.M. Mitigation of Polysulfide Shuttling by Interlayer/Permselective Separators in Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 8095–8129.
- Chong, W.G.; Huang, J.Q.; Xu, Z.L.; Qin, X.Y.; Wang, X.Y.; Kim, J.K. Lithium-Sulfur Battery Cable Made from Ultralight, Flexible Graphene/Carbon Nanotube/Sulfur Composite Fibers. Adv. Funct. Mater. 2017, 27, 1604815.
- Chong, W.G.; Xiao, Y.; Huang, J.-Q.; Yao, S.; Cui, J.; Qin, L.; Gao, C.; Kim, J.-K. Highly conductive porous graphene/sulfur composite ribbon electrodes for flexible lithium-sulfur batteries. Nanoscale 2018, 10, 21132–21141.
- Bruckner, J.; Thieme, S.; Bottger-Hiller, F.; Bauer, I.; Grossmann, H.T.; Strubel, P.; Althues, H.; Spange, S.; Kaskel, S. Carbon- Based Anodes for Lithium Sulfur Full Cells with High Cycle Stability. Adv. Funct. Mater. 2014, 24, 1284–1289.
- Wang, J.; Wu, T.; Zhang, S.; Gu, S.; Jin, J.; Wen, Z. Metal-organic-framework-derived N-C-Co film as a shuttle-suppressing interlayer for lithium sulfur battery. Chem. Eng. J. 2018, 334, 2356–2362.
- Lu, X.; Wang, H.; Liu, X.; Song, Z.; Jiang, N.; Xie, F.; Zheng, Q.; Lin, D. Functional separators prepared via in-situ growth of hollow CoSO4 hydrate arrays on pristine polypropylene membrane for high performance lithium-Sulfur batteries. J. Alloys Compd. 2020, 838, 155618.
- Li, J.; Jiao, C.; Zhu, J.; Zhong, L.; Kang, T.; Aslam, S.; Wang, J.; Zhao, S.; Qiu, Y. Hybrid co-based MOF nanoboxes/CNFs interlayer as microreactors for polysulfides-trapping in lithium-sulfur batteries. J. Energy Chem. 2021, 57, 469–476.
