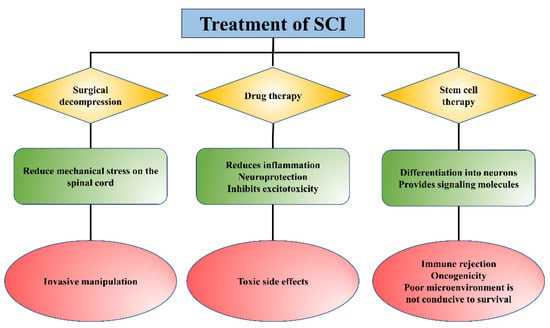
Spinal cord injury (SCI) is a severe neurological injury caused by traffic accidents, trauma, or falls, which leads to significant loss of sensory, motor, and autonomous functions and seriously affects the patient’s life quality. Although considerable progress has been made in mitigating secondary injury and promoting the regeneration/repair of SCI, the therapeutic effects need to be improved due to drug availability. Given their good biocompatibility, biodegradability, and low immunogenicity, injectable hydrogels can be used as delivery systems to achieve controlled release of drugs and other substances (cells and proteins, etc.), offering new hope for SCI repair.
- injectable hydrogel
- spinal cord injury
- delivery systems
1. Introduction
2. Types of Hydrogels
2.1. Natural Hydrogel
2.2. Synthetic Hydrogel
2.3. Composite Hydrogel
3. Application of Hydrogel as a Delivery System in SCI
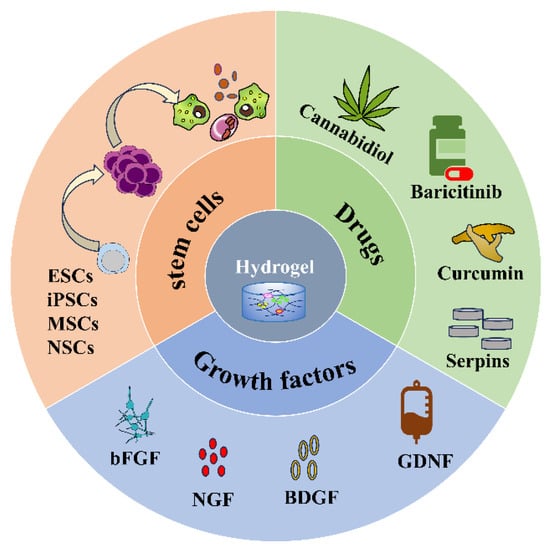
| Encapsulated Substances | Hydrogel Composition | Specific Substances | Function | Ref |
|---|---|---|---|---|
| Stem cells | PLGA; TA; oxidized dextran (Dex) and hyaluronic acid-hydrazide | NSCs | Promote the differentiation of NSCs into neurons while inhibiting the differentiation of astrocytes | [55] |
| HA; collagen | NSCs | Promote NSCs to differentiate into neuron-like cells and play neuroprotective roles | [56] | |
| Gelatin; peroxidase (HRP) and galactose oxidase (GalOx) | MSCs | Enhance the neural differentiation and functional recovery of SCI; | [57] | |
| ECM | Human iPSCs | Reduces inflammation, enhance nerve regeneration, and significantly improve movement | [58] | |
| Gelatin; methacrylic | ADSCs | Promote axon growth, reduce neuroinflammation, and ultimately improve motor function in SCI rats | [59] | |
| Drugs | Alginate; chitosan | Erythropoietin (EPO) | Improve tissue repair and histopathological appearance of the spinal cord at the site of injury | [60] |
| Chitosan; collagen | Serpins | Improve the neurological and motor function and reduce tissue damage caused by in-flammation in SCI rats | [61] | |
| Chitosan | Cannabidiol (CBD) | Reduce apoptosis, improve neurogenesis by enhancing mitochondrial biogenesis | [62] | |
| PLGA; PEG | Baricitinib | Reduce neuronal apoptosis and promote functional recovery in SCI rats | [63] | |
| Chitosan | Curcumin | Favor functional recovery of SCI rats | [64] | |
| Growth factors | Heparin-poloxamer | bFGF and NGF | Improve neuronal survival, inhibit reactive astrogliosis, and promote recovery of motor performance | [65] |
| Alginate | GDNF | Stimulate neurite growth and functional recovery | [66] | |
| Naphthalene acetic acid-phenylalanine-phenylalanine-glycine | Platelet-derived growth factor (PDGF) | Inhibit M1 macrophage infiltration and extrinsic or intrinsic cells apoptosis | [67] | |
| Heparin-Laponite | FGF4 | Inhibit inflammatory response, increase myelination regeneration, and reduce glial/fibrotic scarring | [68] |
3.1. Stem Cells
3.2. Drugs
3.3. Growth Factors
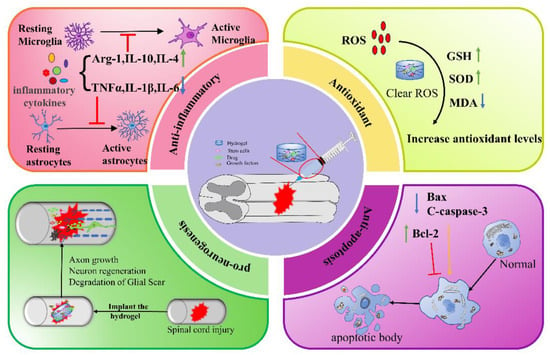
4. Therapeutic Mechanism of Injectable Hydrogels in SCI
4.1. Anti-Inflammation
4.2. Antioxidant
4.3. Anti-Apoptosis
4.4. Pro-Neurogenesis
5. Combination Therapy
6. Conclusions
In summary, natural, synthetic, and composite injectable hydrogels can all be used as delivery systems to encapsulate stem cells, drugs, or GFs for a wide range of applications in SCI therapy. The mechanisms by which hydrogels promote SCI repair include anti-inflammation, anti-oxidation, anti-apoptosis, and pro-neurogenesis (Figure 3), etc. In addition, hydrogel combined with electromagnetic stimulation or phototherapy can also improve the repair of SCI. Although much progress has been made in the study of injectable hydrogels for SCI, there are still certain limitations.
This entry is adapted from the peer-reviewed paper 10.3390/gels9110907
References
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908.
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745.
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533.
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.S.; Claus, C.F.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2020, 43, 425–441.
- Freyermuth-Trujillo, X.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Orozco-Barrios, C.E.; Coyoy-Salgado, A. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells 2022, 11, 2692.
- Sterner, R.C.; Sterner, R.M. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front. Immunol. 2022, 13, 1084101.
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65.
- De Almeida, F.M.; Marques, S.A.; Dos Santos, A.C.R.; Prins, C.A.; Dos Santos Cardoso, F.S.; Dos Santos Heringer, L.; Mendonça, H.R.; Martinez, A.M.B. Molecular approaches for spinal cord injury treatment. Neural Regen. Res. 2023, 18, 23–30.
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet. Neurol. 2022, 21, 659–670.
- Kim, H.N.; McCrea, M.R.; Li, S. Advances in molecular therapies for targeting pathophysiology in spinal cord injury. Expert Opin. Ther. Targets 2023, 27, 171–187.
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151.
- Russo, G.S.; Mangan, J.J.; Galetta, M.S.; Boody, B.; Bronson, W.; Segar, A.; Kepler, C.K.; Kurd, M.F.; Hilibrand, A.S.; Vaccaro, A.R.; et al. Update on Spinal Cord Injury Management. Clin. Spine Surg. 2020, 33, 258–264.
- Rabchevsky, A.G.; Patel, S.P.; Springer, J.E. Pharmacological interventions for spinal cord injury: Where do we stand? How might we step forward? Pharmacol. Ther. 2011, 132, 15–29.
- Cox, A.; Varma, A.; Banik, N. Recent advances in the pharmacologic treatment of spinal cord injury. Metab. Brain Dis. 2015, 30, 473–482.
- Shao, A.; Tu, S.; Lu, J.; Zhang, J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019, 10, 238.
- Gao, L.; Peng, Y.; Xu, W.; He, P.; Li, T.; Lu, X.; Chen, G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int. 2020, 2020, 2853650.
- Kiyotake, E.A.; Martin, M.D.; Detamore, M.S. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022, 139, 43–64.
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698.
- Wang, X.; Wang, Q. Enzyme-Laden Bioactive Hydrogel for Biocatalytic Monitoring and Regulation. Acc. Chem. Res. 2021, 54, 1274–1287.
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426.
- Lv, Z.; Dong, C.; Zhang, T.; Zhang, S. Hydrogels in Spinal Cord Injury Repair: A Review. Front. Bioeng. Biotechnol. 2022, 10, 931800.
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 2022, 234, 108043.
- Liu, W.; Xu, B.; Zhao, S.; Han, S.; Quan, R.; Liu, W.; Ji, C.; Chen, B.; Xiao, Z.; Yin, M.; et al. Spinal cord tissue engineering via covalent interaction between biomaterials and cells. Sci. Adv. 2023, 9, eade8829.
- Ma, T.; Wu, J.; Mu, J.; Gao, J. Biomaterials reinforced MSCs transplantation for spinal cord injury repair. Asian J. Pharm. Sci. 2022, 17, 4–19.
- Assunção-Silva, R.C.; Gomes, E.D.; Sousa, N.; Silva, N.A.; Salgado, A.J. Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration. Stem Cells Int. 2015, 2015, 948040.
- Wang, Y.; Lv, H.Q.; Chao, X.; Xu, W.X.; Liu, Y.; Ling, G.X.; Zhang, P. Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil. Med. Res. 2022, 9, 16.
- Wang, Y.; Tan, H.; Hui, X. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. BioMed Res. Int. 2018, 2018, 7848901.
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128.
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The Application of Hydrogels Based on Natural Polymers for Tissue Engineering. Curr. Med. Chem. 2020, 27, 2658–2680.
- Kushchayev, S.V.; Giers, M.B.; Hom Eng, D.; Martirosyan, N.L.; Eschbacher, J.M.; Mortazavi, M.M.; Theodore, N.; Panitch, A.; Preul, M.C. Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. J. Neurosurg. Spine 2016, 25, 114–124.
- Li, S.; Ke, Z.; Peng, X.; Fan, P.; Chao, J.; Wu, P.; Xiao, P.; Zhou, Y. Injectable and fast gelling hyaluronate hydrogels with rapid self-healing ability for spinal cord injury repair. Carbohydr. Polym. 2022, 298, 120081.
- Khaing, Z.Z.; Milman, B.D.; Vanscoy, J.E.; Seidlits, S.K.; Grill, R.J.; Schmidt, C.E. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng. 2011, 8, 046033.
- Mneimneh, A.T.; Mehanna, M.M. Collagen-based scaffolds: An auspicious tool to support repair, recovery, and regeneration post spinal cord injury. Int. J. Pharm. 2021, 601, 120559.
- Yang, Y.; Fan, Y.; Zhang, H.; Zhang, Q.; Zhao, Y.; Xiao, Z.; Liu, W.; Chen, B.; Gao, L.; Sun, Z.; et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 2021, 269, 120479.
- Liu, X.; Zhang, L.; Xu, Z.; Xiong, X.; Yu, Y.; Wu, H.; Qiao, H.; Zhong, J.; Zhao, Z.; Dai, J.; et al. A functionalized collagen-I scaffold delivers microRNA 21-loaded exosomes for spinal cord injury repair. Acta Biomater. 2022, 154, 385–400.
- Liu, S.; Xie, Y.Y.; Wang, L.D.; Tai, C.X.; Chen, D.; Mu, D.; Cui, Y.Y.; Wang, B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen. Res. 2021, 16, 2284–2292.
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186.
- Liu, K.; Dong, X.; Wang, Y.; Wu, X.; Dai, H. Dopamine-modified chitosan hydrogel for spinal cord injury. Carbohydr. Polym. 2022, 298, 120047.
- Zhao, C.; Rao, J.S.; Duan, H.; Hao, P.; Shang, J.; Fan, Y.; Zhao, W.; Gao, Y.; Yang, Z.; Sun, Y.E.; et al. Chronic spinal cord injury repair by NT3-chitosan only occurs after clearance of the lesion scar. Signal Transduct. Target. Ther. 2022, 7, 184.
- Xiang, W.; Cao, H.; Tao, H.; Jin, L.; Luo, Y.; Tao, F.; Jiang, T. Applications of chitosan-based biomaterials: From preparation to spinal cord injury neuroprosthetic treatment. Int. J. Biol. Macromol. 2023, 230, 123447.
- Han, S.; Lee, J.Y.; Heo, E.Y.; Kwon, I.K.; Yune, T.Y.; Youn, I. Implantation of a Matrigel-loaded agarose scaffold promotes functional regeneration of axons after spinal cord injury in rat. Biochem. Biophys. Res. Commun. 2018, 496, 785–791.
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2022, 34, e2105063.
- Peng, H.; Liu, Y.; Xiao, F.; Zhang, L.; Li, W.; Wang, B.; Weng, Z.; Liu, Y.; Chen, G. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front. Bioeng. Biotechnol. 2023, 11, 1111882.
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release Off. J. Control. Release Soc. 2022, 351, 215–230.
- Peppas, N.A.; Keys, K.B.; Torres-Lugo, M.; Lowman, A.M. Poly(ethylene glycol)-containing hydrogels in drug delivery. J. Control. Release Off. J. Control. Release Soc. 1999, 62, 81–87.
- Aghaie, T.; Jazayeri, M.H.; Manian, M.; Khani, L.; Erfani, M.; Rezayi, M.; Ferns, G.A.; Avan, A. Gold nanoparticle and polyethylene glycol in neural regeneration in the treatment of neurodegenerative diseases. J. Cell. Biochem. 2019, 120, 2749–2755.
- Estrada, V.; Brazda, N.; Schmitz, C.; Heller, S.; Blazyca, H.; Martini, R.; Müller, H.W. Long-lasting significant functional improvement in chronic severe spinal cord injury following scar resection and polyethylene glycol implantation. Neurobiol. Dis. 2014, 67, 165–179.
- Kong, X.B.; Tang, Q.Y.; Chen, X.Y.; Tu, Y.; Sun, S.Z.; Sun, Z.L. Polyethylene glycol as a promising synthetic material for repair of spinal cord injury. Neural Regen. Res. 2017, 12, 1003–1008.
- Shi, R. Polyethylene glycol repairs membrane damage and enhances functional recovery: A tissue engineering approach to spinal cord injury. Neurosci. Bull. 2013, 29, 460–466.
- Zhang, L.; Han, Q.; Chen, S.; Suo, D.; Zhang, L.; Li, G.; Zhao, X.; Yang, Y. Soft hydrogel promotes dorsal root ganglion by upregulating gene expression of Ntn4 and Unc5B. Colloids Surf. B Biointerfaces 2021, 199, 111503.
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501.
- Jiang, F.X.; Yurke, B.; Firestein, B.L.; Langrana, N.A. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann. Biomed. Eng. 2008, 36, 1565–1579.
- Zhao, X.; Lu, X.; Li, K.; Song, S.; Luo, Z.; Zheng, C.; Yang, C.; Wang, X.; Wang, L.; Tang, Y.; et al. Double crosslinked biomimetic composite hydrogels containing topographical cues and WAY-316606 induce neural tissue regeneration and functional recovery after spinal cord injury. Bioact. Mater. 2023, 24, 331–345.
- Man, W.; Yang, S.; Cao, Z.; Lu, J.; Kong, X.; Sun, X.; Zhao, L.; Guo, Y.; Yao, S.; Wang, G.; et al. A multi-modal delivery strategy for spinal cord regeneration using a composite hydrogel presenting biophysical and biochemical cues synergistically. Biomaterials 2021, 276, 120971.
- Huang, F.; Chen, T.; Chang, J.; Zhang, C.; Liao, F.; Wu, L.; Wang, W.; Yin, Z. A conductive dual-network hydrogel composed of oxidized dextran and hyaluronic-hydrazide as BDNF delivery systems for potential spinal cord injury repair. Int. J. Biol. Macromol. 2021, 167, 434–445.
- Duan, H.; Li, X.; Wang, C.; Hao, P.; Song, W.; Li, M.; Zhao, W.; Gao, Y.; Yang, Z. Functional hyaluronate collagen scaffolds induce NSCs differentiation into functional neurons in repairing the traumatic brain injury. Acta Biomater. 2016, 45, 182–195.
- Yao, M.; Li, J.; Zhang, J.; Ma, S.; Wang, L.; Gao, F.; Guan, F. Dual-enzymatically cross-linked gelatin hydrogel enhances neural differentiation of human umbilical cord mesenchymal stem cells and functional recovery in experimental murine spinal cord injury. J. Mater. Chem. B 2021, 9, 440–452.
- Wertheim, L.; Edri, R.; Goldshmit, Y.; Kagan, T.; Noor, N.; Ruban, A.; Shapira, A.; Gat-Viks, I.; Assaf, Y.; Dvir, T. Regenerating the Injured Spinal Cord at the Chronic Phase by Engineered iPSCs-Derived 3D Neuronal Networks. Adv. Sci. 2022, 9, e2105694.
- Yuan, X.; Yuan, W.; Ding, L.; Shi, M.; Luo, L.; Wan, Y.; Oh, J.; Zhou, Y.; Bian, L.; Deng, D.Y.B. Cell-adaptable dynamic hydrogel reinforced with stem cells improves the functional repair of spinal cord injury by alleviating neuroinflammation. Biomaterials 2021, 279, 121190.
- Gholami, M.; Gilanpour, H.; Sadeghinezhad, J.; Asghari, A. Facile fabrication of an erythropoietin-alginate/chitosan hydrogel and evaluation of its local therapeutic effects on spinal cord injury in rats. DARU J. Fac. Pharm. Tehran Univ. Med. Sci. 2021, 29, 255–265.
- Kwiecien, J.M.; Zhang, L.; Yaron, J.R.; Schutz, L.N.; Kwiecien-Delaney, C.J.; Awo, E.A.; Burgin, M.; Dabrowski, W.; Lucas, A.R. Local Serpin Treatment via Chitosan-Collagen Hydrogel after Spinal Cord Injury Reduces Tissue Damage and Improves Neurologic Function. J. Clin. Med. 2020, 9, 1221.
- Zhang, H.; Hu, T.; Xiong, M.; Li, S.; Li, W.X.; Liu, J.; Zhou, X.; Qi, J.; Jiang, G.B. Cannabidiol-loaded injectable chitosan-based hydrogels promote spinal cord injury repair by enhancing mitochondrial biogenesis. Int. J. Biol. Macromol. 2022, 221, 1259–1270.
- Zheng, X.Q.; Huang, J.F.; Lin, J.L.; Zhu, Y.X.; Wang, M.Q.; Guo, M.L.; Zan, X.J.; Wu, A.M. Controlled release of baricitinib from a thermos-responsive hydrogel system inhibits inflammation by suppressing JAK2/STAT3 pathway in acute spinal cord injury. Colloids Surf. B Biointerfaces 2021, 199, 111532.
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021, 6, 4816–4829.
- Hu, X.; Li, R.; Wu, Y.; Li, Y.; Zhong, X.; Zhang, G.; Kang, Y.; Liu, S.; Xie, L.; Ye, J.; et al. Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury. J. Cell. Mol. Med. 2020, 24, 8166–8178.
- Ansorena, E.; De Berdt, P.; Ucakar, B.; Simón-Yarza, T.; Jacobs, D.; Schakman, O.; Jankovski, A.; Deumens, R.; Blanco-Prieto, M.J.; Préat, V.; et al. Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury. Int. J. Pharm. 2013, 455, 148–158.
- Wu, W.; Jia, S.; Xu, H.; Gao, Z.; Wang, Z.; Lu, B.; Ai, Y.; Liu, Y.; Liu, R.; Yang, T.; et al. Supramolecular Hydrogel Microspheres of Platelet-Derived Growth Factor Mimetic Peptide Promote Recovery from Spinal Cord Injury. ACS Nano 2023, 17, 3818–3837.
- Wang, C.; Gong, Z.; Huang, X.; Wang, J.; Xia, K.; Ying, L.; Shu, J.; Yu, C.; Zhou, X.; Li, F.; et al. An injectable heparin-Laponite hydrogel bridge FGF4 for spinal cord injury by stabilizing microtubule and improving mitochondrial function. Theranostics 2019, 9, 7016–7032.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Hashimoto, S.; Nagoshi, N.; Shinozaki, M.; Nakanishi, K.; Suematsu, Y.; Shibata, T.; Kawai, M.; Kitagawa, T.; Ago, K.; Kamata, Y.; et al. Microenvironmental modulation in tandem with human stem cell transplantation enhances functional recovery after chronic complete spinal cord injury. Biomaterials 2023, 295, 122002.
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866.
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel Encapsulation of Mesenchymal Stem Cells and Their Derived Exosomes for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 684.
- Jin, L.Y.; Li, J.; Wang, K.F.; Xia, W.W.; Zhu, Z.Q.; Wang, C.R.; Li, X.F.; Liu, H.Y. Blood-Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2021, 38, 1203–1224.
- Gerndt, S.J.; Rodriguez, J.L.; Pawlik, J.W.; Taheri, P.A.; Wahl, W.L.; Micheals, A.J.; Papadopoulos, S.M. Consequences of high-dose steroid therapy for acute spinal cord injury. J. Trauma 1997, 42, 279–284.
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels-Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371.
- Singh, P.L.; Agarwal, N.; Barrese, J.C.; Heary, R.F. Current therapeutic strategies for inflammation following traumatic spinal cord injury. Neural Regen. Res. 2012, 7, 1812–1821.
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327.
- Lauzon, M.A.; Daviau, A.; Marcos, B.; Faucheux, N. Growth factor treatment to overcome Alzheimer’s dysfunctional signaling. Cell. Signal. 2015, 27, 1025–1038.
- Liu, S.M.; Xiao, Z.F.; Li, X.; Zhao, Y.N.; Wu, X.M.; Han, J.; Chen, B.; Li, J.Y.; Fan, C.X.; Xu, B.; et al. Vascular endothelial growth factor activates neural stem cells through epidermal growth factor receptor signal after spinal cord injury. CNS Neurosci. Ther. 2019, 25, 375–385.
- Shan, B.H.; Wu, F.G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2023, e2210707.
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364.
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflam. 2021, 18, 284.
- David, S.; López-Vales, R.; Wee Yong, V. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. Handb. Clin. Neurol. 2012, 109, 485–502.
- Liu, X.; Zhang, Y.; Wang, Y.; Qian, T. Inflammatory Response to Spinal Cord Injury and Its Treatment. World Neurosurg. 2021, 155, 19–31.
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurother. J. Am. Soc. Exp. Neuro Ther. 2018, 15, 541–553.
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 16–35.
- Chio, J.C.T.; Punjani, N.; Hejrati, N.; Zavvarian, M.M.; Hong, J.; Fehlings, M.G. Extracellular Matrix and Oxidative Stress Following Traumatic Spinal Cord Injury: Physiological and Pathophysiological Roles and Opportunities for Therapeutic Intervention. Antioxid. Redox Signal. 2022, 37, 184–207.
- Fan, B.; Wei, Z.; Feng, S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 2022, 10, 35.
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO(2) Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293.
- Liu, Z.; Guo, S.; Dong, L.; Wu, P.; Li, K.; Li, X.; Li, X.; Qian, H.; Fu, Q. A tannic acid doped hydrogel with small extracellular vesicles derived from mesenchymal stem cells promotes spinal cord repair by regulating reactive oxygen species microenvironment. Mater. Today. Bio 2022, 16, 100425.
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121.
- Basnak’ian, A.G.; Baskov, A.V.; Sokolov, N.N.; Borshchenko, I.A. Apoptosis during spinal cord trauma: Prospects for pharmacological correction. Vopr. Meditsinskoi Khimii 2000, 46, 431–443.
- Robertson, G.S.; Crocker, S.J.; Nicholson, D.W.; Schulz, J.B. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000, 10, 283–292.
- Li, X.; Duan, L.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels 2022, 8, 482.
- Li, Y.; Yang, L.; Hu, F.; Xu, J.; Ye, J.; Liu, S.; Wang, L.; Zhuo, M.; Ran, B.; Zhang, H.; et al. Novel Thermosensitive Hydrogel Promotes Spinal Cord Repair by Regulating Mitochondrial Function. ACS Appl. Mater. Interfaces 2022, 14, 25155–25172.
- Kim, M.S.; Lee, H.B. Perspectives on tissue-engineered nerve regeneration for the treatment of spinal cord injury. Tissue Eng. Part A 2014, 20, 1781–1783.
- Zheng, B.; Tuszynski, M.H. Regulation of axonal regeneration after mammalian spinal cord injury. Nat. Rev. Mol. Cell Biol. 2023, 24, 396–413.
- Han, Q.; Xie, Y.; Ordaz, J.D.; Huh, A.J.; Huang, N.; Wu, W.; Liu, N.; Chamberlain, K.A.; Sheng, Z.H.; Xu, X.M. Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Cell Metab. 2020, 31, 623–641.e628.
- Morgado, P.I.; Palacios, M.; Larrain, J. In situ injectable hydrogels for spinal cord regeneration: Advances from the last 10 years. Biomed. Phys. Eng. Express 2019, 6, 012002.
- Curcio, M.; Bradke, F. Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annu. Rev. Cell Dev. Biol. 2018, 34, 495–521.
- Zhang, H.; Xu, J.; Saijilafu. The effects of GelMA hydrogel on nerve repair and regeneration in mice with spinal cord injury. Ann. Transl. Med. 2021, 9, 1147.
- Wang, C.; Liu, Y.; Wang, Y.; Wei, Z.; Suo, D.; Ning, G.; Wu, Q.; Feng, S.; Wan, C. Low-frequency pulsed electromagnetic field promotes functional recovery, reduces inflammation and oxidative stress, and enhances HSP70 expression following spinal cord injury. Mol. Med. Rep. 2019, 19, 1687–1693.
- Yang, B.; Liang, C.; Chen, D.; Cheng, F.; Zhang, Y.; Wang, S.; Shu, J.; Huang, X.; Wang, J.; Xia, K.; et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact. Mater. 2022, 15, 103–119.
- Liu, W.; Luo, Y.; Ning, C.; Zhang, W.; Zhang, Q.; Zou, H.; Fu, C. Thermo-sensitive electroactive hydrogel combined with electrical stimulation for repair of spinal cord injury. J. Nanobiotechnol. 2021, 19, 286.
- Bhattacharyya, S.; Dinda, A.; Vishnubhatla, S.; Anwar, M.F.; Jain, S. A combinatorial approach to modulate microenvironment toward regeneration and repair after spinal cord injury in rats. Neurosci. Lett. 2021, 741, 135500.
