2. JAK/STAT Pathway
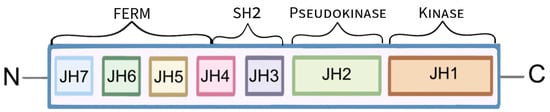
JAKs are a family of cytoplasmic, non-receptor tyrosine kinases that are composed of seven JAK homology (JH) domains. Functionally, a distinction is made between the FERM domain (JH4, 5, 6 and 7), the Src homology 2 (SH2) domain (JH3 and 4) and the tandem kinase domains: pseudokinase (JH2) and tyrosine kinase (JH1) (
Figure 1) [
9,
10]. The tyrosine kinase domain consists of approximately 250 amino acid residues. JH1 encodes the kinase protein, which is the structure domain of the kinase. It is responsible for substrate phosphorylation, and it is this domain that has become the main target for the introduction of new drug therapies. The pseudokinase domain resembles the kinase domain in its structure but does not exhibit tyrosine kinase activity. The pseudokinase domain is involved in the interaction of JAK and STAT and the inhibition of tyrosine kinase activity by binding to it. The function of the SH2 and FERM domains is to mediate interactions with two intracellular peptide motifs of the cytokine receptor: the proline rich ‘Box1’ and the hydrophobic ‘Box2’ [
9]. There are four different Janus kinases: JAK1, JAK2, JAK3 and TYK2 (tyrosine kinase 2) [
6,
11,
12]. Expression of JAK1, JAK2 and TYK2 occurs in many tissues to regulate immunity, while JAK3 is expressed mainly in hematopoietic cells participating in hematopoiesis [
13,
14,
15,
16]. The action of JAK is strictly determined by the mediators of inflammation–cytokines: interleukins (IL), interferons (IFN), growth factors along with their receptors with which JAKi are linked [
6,
14,
17,
18]. Cytokine-induced signal transport is mediated by different combinations of different types of JAK proteins, for example, the combination of JAK2 and TYK2 is necessary for the action of IL-12 and Il-23 [
5,
14]. Cytokines bind to the extracellular domains of corresponding receptors located on specific cells leading to conformational changes within the intracellular domain. This results in bringing two JAK molecules close enough to each other that their mutual phosphorylation and activation is feasible [
6,
11,
17]. The activated JAKi then lead to further intracellular signal transduction through phosphorylation and activation of STAT proteins [
12]. STAT proteins are signal transducers and activators of transcription that are intracellular transcription factors. The family of these proteins includes seven members: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6 [
13,
19]. STATs are involved in many key cellular processes: processes of proliferation, differentiation, apoptosis and functional activation [
19,
20]. These proteins are composed of an N-terminal domain, a coiled-coil-type domain, a DNA-binding domain, a transcription activation domain, an SH2 domain and a tyrosine activation domain [
21]. Activated STAT proteins dimerize and are transported into the cell nucleus to positively or negatively modulate the expression of target genes, encoding, for example, inflammatory cytokines involved in the formation of numerous diseases, including dermatological conditions [
13,
22].
Figure 1. Schematic presentation of the Janus kinase’s structure. The function of the FERM and SH2 domains is to link JAK to receptors. The pseudokinase domain is thought to regulate the activity of the kinase domain, which leads to the phosphorylation of the receptor tyrosine, followed by phosphorylation of downstream molecules.
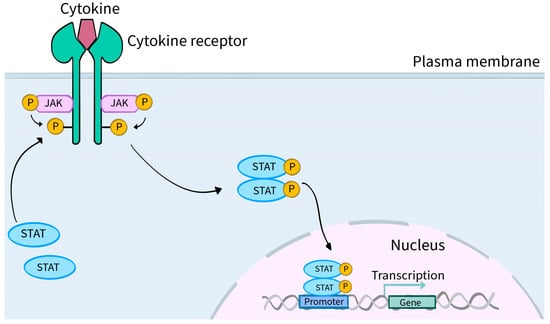
In general, the JAK-STAT pathway is a pathway activated by cytokine stimulation that allows signals from outside the cell to pass through the cell membrane to the nucleus, resulting in changes in DNA transcription [
14].
Figure 2 shows a schematic presentation of JAK-STAT pathway. The utilization of JAK by various receptors coupled to downstream STAT signal transduction results in a mechanism to achieve exceptional in vivo specificity for more than 60 cytokines and growth factors [
11,
23].
Figure 2. Schematic presentation of JAK-STAT pathway. The attachment of a ligand in the form of a cytokine or hormone (examples: IFN, IL-2, IL-27, IL-19, EPO and OSM) to the extracellular domain of the respective receptors located on specific cells induces conformational changes within their intracellular parts. These changes lead to the two JAK molecules approaching each other, resulting in their phosphorylation (P) and activation. Phosphorylation of the cytoplasmic part of the receptor also occurs, creating a docking site for STAT proteins. STAT proteins, which are signal transducers and activators of transcription, are intracellular transcription factors. STATs bind to the cytoplasmic part of the receptor and their phosphorylation, activation and dimerization occur. A dimer consisting of two STAT molecules translocates into the cell nucleus, where it directly interacts with the DNA matrix and positively or negatively regulates the expression of thousands of different target genes, encoding, for example, inflammatory cytokines that are involved in the pathogenesis of numerous diseases, including dermatological conditions.
3. Janus Kinase Inhibitors
Recognition of the importance of the JAK/STAT pathway in the pathogenesis of many inflammatory and autoimmune diseases has contributed to the development of a new class of drugs—Janus kinase inhibitors. JAKi stop the intracellular signal transduction pathway by inhibiting JAK protein phosphorylation catalyzed by the kinase component of JAK [
4]. In September 2021, the Food and Drug Administration (FDA) approved the first JAK inhibitor, ruxolitinib, for the treatment of skin disorders [
24]. Since then, more Janus kinase inhibitors have been successively approved for the treatment of dermatoses. The advantage of JAKinibs is that they can be administered by oral or topical routes. This distinguishes them from biologic drugs, which are administered via subcutaneous or intravenous injections. Topical application of JAKi can successfully reduce the risk of side effects compared to their use via the oral route. Noteworthy is the fact that, unlike topical corticosteroids, topical JAKinibs do not cause telangiectasia or skin atrophy [
1]. There are two generations of JAKi. Generation I, which includes, for example, ruxolitinib or baricitinib, is characterized by lower specificity toward various Janus kinase isoforms, which is associated with a relatively higher risk of side effects. However, their use can be argued by the theory that blocking multiple JAKi benefits therapeutic success. Second-generation JAKinibs (for example, upadacitinib, abrocitinib, deucravacitinib) are characterized by greater selectivity and specificity. This causes them to be more valued, as their use results in fewer side effects which has an impact on the eventual maintenance of treatment efficacy [
4,
5,
25,
26]. Currently, atopic dermatitis, alopecia areata, vitiligo and psoriasis are dermatological conditions for the treatment of which JAKi have been officially approved by the FDA or EMA.
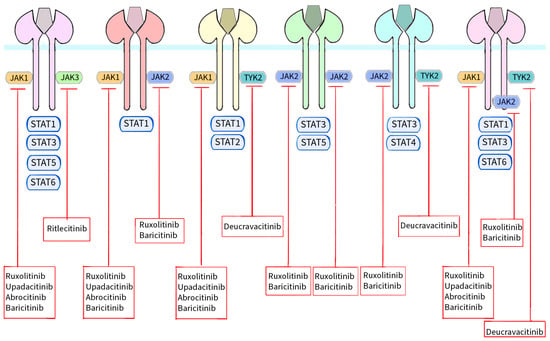
Figure 3 shows a schematic presentation of Janus kinases together with the STAT proteins with which they interact and the site of action of individual Janus kinase inhibitors [
10,
12].
Figure 3. Schematic representation of JAKs with their respective STAT proteins and the site of action of individual JAKs approved by the FDA or EMA for use in the treatment of dermatological conditions. The binding of different ligands to their specific receptor subunits leads to the activation of a specific JAK/STAT pathway. Receptors for cytokines transmit the signal to the cell nucleus via their associated Janus kinases. There are four enzymes in this family: JAK1, JAK2, JAK3 and TYK2. These kinases are essential for signal transduction from cytokine receptors lacking kinase activity. Signal transducers and activators of STAT transcription are also involved in signal transport to the cell nucleus. Seven homologous STAT proteins are currently known: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6. Each cytokine receptor recruits and activates a specific combination in the JAK/STAT cascades as shown in the figure. Activation results in signal transduction to the cell nucleus, modulation of gene expression and formation of molecules that may be involved in the pathogenesis of skin diseases. However, the signal transduction cascade from the receptor, through JAK/STAT to the cell nucleus, is inhibited by Janus kinase inhibitors. Ruxolitinib and baricitinib are inhibitors of both JAK1 and JAK2, upadacitinib and abrocitinib inhibit JAK1, ritlecitinib blocks JAK3 activity and deucravacitinib inhibits TYK2.
4. Dermatological Conditions Where JAK Inhibitors Are Approved by the FDA or EMA
4.1. Atopic Dermatitis
Atopic dermatitis (AD) is one of the chronic inflammatory dermatoses, with genetic predisposition, abnormal skin barrier function, abnormal microbiome, dysfunctional immune system and environmental factors cited as underlying causes [
29]. Chronic, persistent pruritus can significantly reduce a patient’s quality of life or self-esteem, increase the risk of depression or anxiety, and have a negative impact on sleep [
30,
31]. The diagnosis of AD is relatively more common in the pediatric population, but this skin disease can occur at any age [
1]. A key role in the pathogenesis of AD is attributed to a strong activation of the immune response, both in the serum and in the skin, involving Th2 helper lymphocytes with their associated cytokines IL-4, IL-5, IL-13 and IL-31. The cytokines IL-4, IL-13 and IL-31 require further signaling through the JAK/STAT pathway [
32].
Inhibition of gene expression for filaggrin, involucrin and loricrin via IL-4 and IL-13 promotes skin dehydration and destabilizes skin barrier integrity resulting in dryness and increased likelihood of skin superinfection [
38,
39]. In addition, modulation of gene expression for cathelicidin and β-defensins (innate immune response genes) potentiates the risk of skin infection by pathogens. This results in exacerbation of AD [
36].
It is noteworthy that Th1 lymphocytes are also involved in the pathogenesis of AD along with the cytokine it produces, IFN-γ, and Th17/Th22 lymphocytes along with IL-17 or IL-22. IL-22 plays a role especially in chronic lesions by promoting epidermal hyperplasia [
12]. These interleukins also act in a JAK-STAT pathway-dependent manner [
33]. Ruxolitinib, upadicitinib, abrocitinib and baricitinib are JAKinibs approved by the FDA or EMA for the treatment of AD.
Ruxolitinib belongs to the first-generation JAKinibs that inhibit JAK1 and JAK2. Two phase 3 trials (this study is registered at
ClinicalTrials.gov available online:
https://www.clinicaltrials.gov/ (accessed on 2 October 2023)), NCT03745638, NCT03745651) have confirmed the efficacy and safety of ruxolitinib cream in AD in monotherapy. It is recommended to be used continuously for 8 weeks twice daily, and then after continuous treatment, it should be used occasionally as needed for long-term disease control. The low plasma concentration of ruxolitinib suggests that systemic JAK inhibition is highly unlikely in this case. Adverse effects occurred relatively infrequently and were mostly unrelated to treatment [
40].
Upadicitinib is a second-generation JAKinib, inhibiting JAK1. Two replicated, randomized, double-blind, controlled phase 3 studies (NCT03569293 and NCT03607422) showed that the use of one upadicitinib tablet per day as a monotherapy is an effective treatment for adolescents and adults with moderate to severe atopic dermatitis in terms of skin symptoms, itching, skin pain and quality of life [
41]. In contrast, another phase 3 study (NCT04195698) showed that patients previously treated with dupilumab had more favorable treatment outcomes after changing it to upadicitinib [
42]. Upadicitinib has no new side effects compared to other JAK inhibitors, and its safety profile is reasonably acceptable (NCT03569293, NCT03607422, NCT03568318) [
43].
Abrocitinib is a second-generation JAK1 inhibitor used for atopic dermatitis (moderate to severe) in the form of 100 mg or 200 mg tablets (one tablet per day). Observations made during the Phase 3 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN trial (NCT03627767) showed that continuous monotherapy with abrocitinib 200 mg is the therapy with the best results in terms of maintaining disease control. No exacerbation of symptoms occurred in patients treated with the 100 mg dose for the 40 weeks of the trial, so it is believed that induction-maintenance therapy (using abrocitinib 200 mg first and then switching to the 100 mg dose) will be the most rational approach among most patients. On the other hand, in case of possible AD exacerbation during abrocitinib therapy, combination therapy is recommended: abrocitinib 200 mg combined with a topical corticosteroid [
44]. Abrocitinib shows superiority over dupilumab, with faster and greater improvement in skin clearance (NCT03720470) [
45,
46].
Baricitinib is a JAK1 and JAK2 inhibitor. The use of baricitinib in monotherapy at a dose of 4 mg or a reduced dose of 2 mg reduces pruritus, improves skin, sleep and quality of life among patients struggling with moderate to severe atopic dermatitis (NCT03334435) (NCT03334435) [
47,
48,
49]. For baricitinib, the incidence of adverse events of special interest (AESI) is low [
50].
4.2. Alopecia Areata
Alopecia areata (AA) is characterized by partial or complete, sudden, non-scarring hair loss with preservation of hair follicles. The incidence ranges from 1.7 to 2.1%, and the first symptoms usually occur before the age of 30 [
51,
52]. The disease can affect all human hair areas (in both children and adults). AA can be associated with psychological suffering for the patient and a decrease in quality of life, especially when it affects areas such as the scalp, beard, mustache, eyelashes, or eyebrows [
53]. Alopecia areata arises from loss of immune privileging in hair follicles during the anagen phase and results in their attack by autoreactive CD8+ T cells and NK T cells [
54]. Follicles in AA are characterized by increased expression of MHC class I, MHC class II, elevated levels of IL-2, IL-15 and CXCL belonging to the pro-inflammatory interleukin family, and abundant infiltration of various inflammatory cells [
55,
56]. CD8+ T lymphocytes, upon activation by NK cells via the NKG2D receptor, produce IFNγ mediated by JAK1 and JAK3. Interferon stimulates IL-15 secretion via follicular epithelial cells using JAK1 and JAK2 signaling. Interleukin-15 affects CD8+ T lymphocytes, also through the JAK-STAT pathway, resulting in the secretion of perforin and granzymes by these lymphocytes. The result of these processes is hair follicle dystrophy and premature onset of the catagen phase resulting in alopecia [
57,
58,
59].
Janus kinase inhibitors are a kind of breakthrough in the treatment of alopecia areata. Baricitinib and ritlecitinib are the first and, so far, only drugs approved by the FDA for the treatment of AA. Baricitinib has found use for treating the disease among adult patients (≥18 yo), while ritlecitinib can be used in both adult and adolescent patients (≥12 yo). It is noteworthy that the research on these two formulations was conducted by a single doctor—Dr. Brett King from Yale School of Medicine [
60].
Baricitinib is a first-generation JAKinib that inhibits JAK1 and JAK2 [
4]. Two randomized, placebo-controlled phase 3 trials conducted by a team led by Dr. Brett King showed that oral baricitinib administered once daily had hair regrowth efficacy compared to the control group after 36 weeks of use. The percentage of patients with a SALT score ≤20 at 36 weeks of use in the BRAVE-AA1 trial (NCT03570749) was 38.8% for the 4 mg dose of baricitinib, 22.8% for the 2 mg drug and 6.2% for placebo, and for the BRAVE-AA2 trial (NCT03899259) the percentages were 35.9%, 19.4% and 3.3%, respectively. Acne, increased cholesterol and creatine kinase levels were relatively more common with baricitinib than placebo [
61].
Ritlecitinib belongs to the second-generation inhibitors that irreversibly inhibit JAK3 [
62]. A formulation containing this active ingredient was relatively recently approved for the treatment of AA: the FDA approved it in June 2023 and the EMA in September 2023. A phase 3 trial lasting 48 weeks, also supervised by Dr. King, showed ritlecitinib to be effective in treating AA and well tolerated among the population aged 12 years and older. Doses of 30 mg and 50 mg taken once daily (with or without a saturating dose of 200 mg taken over four weeks) resulted in significant hair regrowth compared with the control group. The drug was generally safe, and major adverse cardiovascular events, opportunistic infections or deaths were reported throughout the study period (NCT03732807) [
63]. A long-term evaluation of ritlecitinib is underway: NCT04006457.
4.3. Non-Segmental Vitiligo
Acquired vitiligo involves the formation of well-demarcated, discolored patches on the skin of any part of the body as a result of the loss of melanocytes within the epidermis. This dermatosis affects about 1–2% of the human population. Non-segmental vitiligo clinically occupies the skin surface regardless of the dermatomes. Skin lesions in the course of vitiligo impinge on the patient’s quality of life, leading to psychic discomfort, social withdrawal and stigmatization [
64,
65,
66,
67,
68]. Certain exogenous and/or endogenous factors in genetically predisposed individuals lead to cellular stress within melanocytes, which promotes the migration of CD8+ T lymphocytes into the epidermis. CD8+ T lymphocytes are responsible for perforin- and granzyme-mediated destruction of melanocytes. These lymphocytes are also responsible for the local production of disease-promoting proteins: interferon gamma and tumor necrosis factor alpha. IFN-γ causes activation of the JAK/STAT pathway in nearby keratinocytes leading to increased levels of the chemokines CXCL9 and CXCL10. It is worth noting that CXCL10 binds to the CXCR3 receptor located on CD8+ T cells—an example of positive feedback. The CXCL10/CXCR3 axis is involved in recruiting more T cells to the skin, exacerbating inflammation. Interferon-gamma is responsible for inhibiting melanogenesis and inducing melanocyte apoptosis. IFN-γ, along with its associated heterodimer: JAK1-JAK2, plays an important role in the pathogenesis of vitiligo [
69,
70,
71,
72,
73].
Ruxolitinib is the first and only FDA-approved pharmacological drug for the treatment of non-segmental vitiligo. It belongs to the first generation JAK1 and JAK2 inhibitors. Two randomized phase 3 trials (NCT04052425 and NCT04057573) were conducted in which patients in the study group were applied 1.5% ruxolitinib cream twice daily for 52 weeks. This ultimately resulted in relatively greater repigmentation of lesions compared to the control group. However, it is noteworthy that patients developed acne and pruritus at the application site [
74,
75].
4.4. Psoriasis
Psoriasis (PsO) is an inflammatory erythematous and scaly skin disease that affects about 2% of the population. It has been recognized by the World Health Organization as a serious non-communicable disease, and the continued increase in its incidence is a public health concern. The course of ordinary (plaque-like) PsO results in characteristic sharply demarcated erythematous, itchy and scaly lesions [
23,
76,
77,
78]. PsO is characterized by the properties of an autoimmune disease on (auto)inflammatory grounds [
79] Activated myeloid dendritic cells secrete TNF-α, IL-23 and IL-12, the latter two interleukins affecting Th17 and Th1 proliferation. This results in an accumulation of Th17 and Th1 lymphocytes within the lesions and their secretion of IL-17, IL-21 and IL-22 (Th17) and IFNγ (Th1). It is worth noting that IL-23, for example, promotes Th17 proliferation precisely through JAK1/JAK2/TYK2 signaling. Finally, IL-22, after binding to the surface receptors IL-10R2 and IL-22R1, leads to acanthosis of keratinocytes also through the JAK/STAT pathway, more specifically with the participation of JAK1/TYK2 and STAT3. In addition, IL-21 and IL-6, which are present around psoriatic lesions, stimulate Th-17 to produce IL-17 through a JAK-STAT signaling-dependent pathway [
80,
81,
82,
83,
84,
85].
Deucravacitinib is a TYK2 inhibitor approved by the FDA and EMA for the treatment of PsO. In the randomized phase 3 PETYK PSO-1 trial (NCT03624127), participants were assigned to a group receiving deucravacitinib 6 mg once daily, to a group receiving apremilast 30 mg daily, or to a placebo group. At week 16, the response rate for PASI 75 was relatively higher for the deucravacitinib-treated group than for the apremilast-treated group and the placebo group, 58.4%, 35.1% and 12.7%, respectively. Efficacy was maintained until the 52nd week of the study. The most common side effects among patients using deucravactinib were nasopharyngitis (6.3%) and upper respiratory tract infection (6.3%) [
86].
4.5. JAK Inhibitors in Other Dermatology Conditions
The JAK/STAT pathway is involved in the pathogenesis of many other diseases manifested by skin lesions. Studies are underway to test the therapeutic potential of Janus kinase inhibitors in such dermatological conditions as: hidradenitis suppurativa, chronic hand eczema, diffuse cutaneous systemic scleroderma, granuloma annulare, dermatomyositis, lichen planus and lupus erythematosus.