Proteins and peptides are potential therapeutic agents, but their physiochemical properties make their use as drug substances challenging. Hydrogels are hydrophilic polymeric networks that can swell and retain high amounts of water or biological fluids without being dissolved. Due to their biocompatibility, their porous structure, which enables the transport of various peptides and proteins, and their protective effect against degradation, hydrogels have gained prominence as ideal carriers for these molecules’ delivery. Particularly, stimuli-responsive hydrogels exhibit physicochemical transitions in response to subtle modifications in the surrounding environment, leading to the controlled release of entrapped proteins or peptides.
- stimuli-responsive hydrogels
- proteins
- peptides
- protein delivery
1. Introduction
2. Therapeutic Proteins
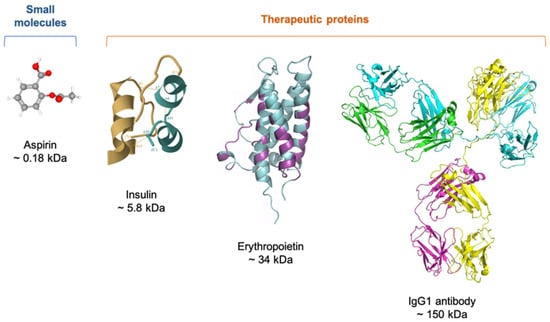
2.1. Characteristics
2.2. Delivery of Therapeutic Proteins
2.2.1. Parenteral Route
2.2.2. Oral Route
2.2.3. Nasal Route
2.2.4. Pulmonary Route
2.2.5. Ocular Route
2.2.6. Transdermal Route
3. Hydrogels
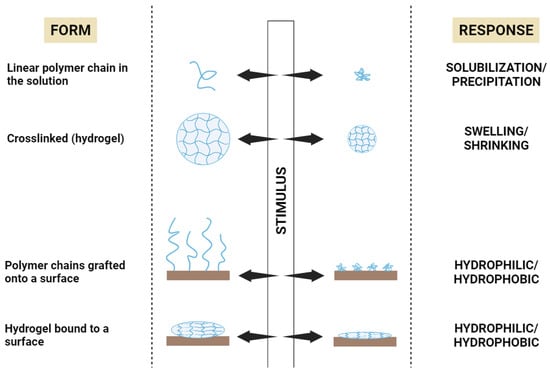
3.1. Definition
3.2. Stimuli-Responsive Polymers
3.2.1. Temperature-Responsive Polymers
3.2.2. pH-Responsive Polymers
3.2.3. Ionic Strength-Responsive Polymers
3.2.4. Biomolecule-Responsive Polymers
3.2.5. Enzyme-Responsive Polymers
3.2.6. Dual and Multiple Stimuli-Responsive Polymers
4. Stimuli-Responsive Hydrogels for Protein Delivery
In a strategy to deal with the problems of protein delivery in the stomach, Lima et al. [78] chose alginate as the hydrogel matrix and bovine serum albumin (BSA) as a model protein. The resulting hydrogel showed biocompatibility and pH-dependent BSA release and swelling profile, reaching the highest value of swelling at pH 7.4. The overall results suggested that the performance of this alginate-based hydrogel as an oral drug delivery system would be excellent.
This entry is adapted from the peer-reviewed paper 10.3390/gels9100802
References
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308.
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145.
- Asfour, M.H. Advanced trends in protein and peptide drug delivery: A special emphasis on aquasomes and microneedles techniques. Drug Deliv. Transl. Res. 2021, 11, 1–23.
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use, 6th ed.; Pharmaceutical Press: London, UK, 2015.
- Lin, J.H. Pharmacokinetics of biotech drugs: Peptides, proteins and monoclonal antibodies. Curr. Drug Metab. 2009, 10, 661–691.
- Irianti, M.K.; Rahmasari, R.; Arifianti, A.E.; Iswandana, R. Non-invasive strategies for protein drug delivery: Oral, transdermal, and pulmonary. J. Appl. Pharm. Sci. 2020, 10, 166–179.
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888.
- Bae, K.H.; Kurisawa, M. Emerging hydrogel designs for controlled protein delivery. Biomater. Sci. 2016, 4, 1184–1192.
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603.
- Vasudevan, D.; Sreekumari, S.; Vaidyanathan, K. Proteins: Structure and Function. In Textbook of Biochemistry for Medical Students; Jaypee Brothers Medical Publishers: New Delhi, India, 2017; pp. 36–51.
- Jain, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 293–329.
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Protein/Peptide Drug Delivery Systems: Practical Considerations in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 651–684.
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39.
- Timofeev, V.; Samygina, V. Protein Crystallography: Achievements and Challenges. Crystals 2023, 13, 71.
- Voet, D.; Voet, J.G. Amino acids. In Biochemistry, 4th ed.; John Wiley & Sons: New York, NY, USA, 2010; pp. 67–78.
- Liang, W.; Pan, H.W.; Vllasaliu, D.; Lam, J.K.W. Pulmonary Delivery of Biological Drugs. Pharmaceutics 2020, 12, 1025.
- Awwad, S.; Angkawinitwong, U. Overview of antibody drug delivery. Pharmaceutics 2018, 10, 83.
- Datta-Mannan, A. Mechanisms Influencing the Pharmacokinetics and Disposition of Monoclonal Antibodies and Peptides. Drug Metab. Dispos. 2019, 47, 1100–1110.
- Joseph, M.; Trinh, H.M.; Mitra, A.K. Peptide and Protein-Based Therapeutic Agents. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 145–167.
- Goldberg, M.; Gomez-Orellana, I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2003, 2, 289–295.
- Vugmeyster, Y.; Xu, X.; Theil, F.P.; Khawli, L.A.; Leach, M.W. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J. Biol. Chem. 2012, 3, 73–92.
- Jain, D.; Mahammad, S.S.; Singh, P.P.; Kodipyaka, R. A review on parenteral delivery of peptides and proteins. Drug Dev. Ind. Pharm. 2019, 45, 1403–1420.
- Ibeanu, N.; Egbu, R.; Onyekuru, L.; Javaheri, H.; Khaw, P.T.; Williams, G.R.; Brocchini, S.; Awwad, S. Injectables and Depots to Prolong Drug Action of Proteins and Peptides. Pharmaceutics 2020, 12, 999.
- Usach, I.; Martinez, R.; Festini, T.; Peris, J.E. Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv. Ther. 2019, 36, 2986–2996.
- Jackisch, C.; Müller, V.; Maintz, C.; Hell, S.; Ataseven, B. Subcutaneous Administration of Monoclonal Antibodies in Oncology. Geburtshilfe Frauenheilkd 2014, 74, 343–349.
- Tomar, D.S.; Kumar, S.; Singh, S.K.; Goswami, S.; Li, L. Molecular basis of high viscosity in concentrated antibody solutions: Strategies for high concentration drug product development. MAbs 2016, 8, 216–228.
- Devadasu, V.R.; Deb, P.K.; Maheshwari, R.; Sharma, P.; Tekade, R.K. Physicochemical, Pharmaceutical, and Biological Considerations in GIT Absorption of Drugs. In Dosage Form Design Consideration; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 149–178.
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B 2019, 9, 902–922.
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248.
- Wright, L.; Barnes, T.J.; Prestidge, C.A. Oral delivery of protein-based therapeutics: Gastroprotective strategies, physiological barriers and in vitro permeability prediction. Int. J. Pharm. 2020, 585, 119488.
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148.
- Linnankoski, J.; Mäkelä, J.; Palmgren, J.; Mauriala, T.; Vedin, C.; Ungell, A.L.; Lazorova, L.; Artursson, P.; Urtti, A.; Yliperttula, M. Paracellular porosity and pore size of the human intestinal epithelium in tissue and cell culture models. J. Pharm. Sci. 2010, 99, 2166–2175.
- Thwala, L.N.; Préat, V.; Csaba, N.S. Emerging delivery platforms for mucosal administration of biopharmaceuticals: A critical update on nasal, pulmonary and oral routes. Expert Opin. Drug Deliv. 2017, 14, 23–36.
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40.
- Balcão, V.; Moutinho, C. Proteins and Peptides: Non-Invasive Delivery. In Encyclopedia of Pharmaceutical Science and Technology; Informa Healthcare: London, UK, 2013; pp. 2555–2578.
- Yoshida, H.; Usui, A.; Abe, Y.; Goda, Y.; Izutsu, K.-I. Relationship Between Geometric and Aerodynamic Particle Size Distributions in the Formulation of Solution and Suspension Metered-Dose Inhalers. AAPS PharmSciTech 2020, 21, 158.
- Patton, J.S.; Brain, J.D.; Davies, L.A.; Fiegel, J.; Gumbleton, M.; Kim, K.J.; Sakagami, M.; Vanbever, R.; Ehrhardt, C. The particle has landed—Characterizing the fate of inhaled pharmaceuticals. J. Aerosol. Med. Pulm. Drug Deliv. 2010, 23, 71–87.
- Matthews, A.A.; Ee, P.L.R.; Ge, R. Developing inhaled protein therapeutics for lung diseases. Mol. Biomed. 2020, 1, 11.
- Jin, L.; Zhou, Q.T.; Chan, H.K.; Larson, I.C.; Pennington, M.W.; Morales, R.A.V.; Boyd, B.J.; Norton, R.S.; Nicolazzo, J.A. Pulmonary Delivery of the Kv1.3-Blocking Peptide HsTX1 for the Treatment of Autoimmune Diseases. J. Pharm. Sci. 2016, 105, 650–656.
- Patton, J.S.; Fishburn, C.S.; Weers, J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 338–344.
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538.
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28.
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64.
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194.
- Chaulagain, B.; Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Passive delivery of protein drugs through transdermal route. Artif. Cells Nanomed. Biotechnol. 2018, 46, 472–487.
- Katikaneni, S. Transdermal delivery of biopharmaceuticals: Dream or reality? Ther. Deliv. 2015, 6, 1109–1116.
- Chatterjee, S.; Hui, P.C.-l. Stimuli-Responsive Hydrogels: An Interdisciplinary Overview. In Hydrogels; Lăcrămioara, P., Mihaela Violeta, G., Cristina-Elena, D.-P., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 2.
- Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O.; et al. A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics 2018, 10, 16.
- Mahlumba, P.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery. Molecules 2016, 21, 1002.
- Chatterjee, S.; Chi-Leung Hui, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547.
- Jocic, D.; Tourrette, A.; Lavric, P.K. Biopolymer-based Stimuli-Responsive Polymeric Systems for Functional Finishing of Textiles. In Biopolymers; Elnashar, M., Ed.; IntechOpen: Rijeka, Croatia, 2010; Chapter 3.
- Sarwan, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. Hybrid Thermo-Responsive Polymer Systems and Their Biomedical Applications. Front. Mater. 2020, 7, 73.
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275.
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Injectable Poloxamer Hydrogels for Local Cancer Therapy. Gels 2023, 9, 593.
- Chatterjee, S.; Hui, P.C. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 86.
- Altomare, L.; Bonetti, L.; Campiglio, C.E.; De Nardo, L.; Draghi, L.; Tana, F.; Farè, S. Biopolymer-based strategies in the design of smart medical devices and artificial organs. Int. J. Artif. Organs 2018, 41, 337–359.
- Zhang, J.; Jiang, X.; Xiang, W.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. J. Phys. Mater. 2019, 2, 032004.
- Zha, L.; Banik, B.; Alexis, F. Stimulus responsive nanogels for drug delivery. Soft Matter 2011, 7, 5908–5916.
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164.
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137.
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228.
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133.
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Doghish, A.S.; Tanaka, R. Stimuli-responsive hydrogels: Smart state of-the-art platforms for cardiac tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1174075.
- Feng, Y.; Taraban, M.; Yu, Y.B. The Effect of Ionic Strength on the Mechanical, Structural and Transport Properties of Peptide Hydrogels. Soft Matter 2012, 8, 11723–11731.
- Sharifzadeh, G.; Hosseinkhani, H. Biomolecule-Responsive Hydrogels in Medicine. Adv. Healthc. Mater. 2017, 6, 1700801.
- Cao, J.; Yuan, P.; Wu, B.; Liu, Y.; Hu, C. Advances in the Research and Application of Smart-Responsive Hydrogels in Disease Treatment. Gels 2023, 9, 662.
- Zhao, L.; Wang, L.; Zhang, Y.; Xiao, S.; Bi, F.; Zhao, J.; Gai, G.; Ding, J. Glucose Oxidase-Based Glucose-Sensitive Drug Delivery for Diabetes Treatment. Polymers 2017, 9, 255.
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323.
- Honey Priya, J.; Rijo, J.; Anju, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127.
- Lim, S.L.; Ooi, C.-W.; Low, L.E.; Tan, W.S.; Chan, E.-S.; Ho, K.L.; Tey, B.T. Synthesis of poly(acrylamide)-based hydrogel for bio-sensing of hepatitis B core antigen. Mater. Chem. Phys. 2020, 243, 122578.
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319.
- Zhao, Y.; Ran, B.; Xie, X.; Gu, W.; Ye, X.; Liao, J. Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy. Gels 2022, 8, 741.
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979.
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724.
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107.
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657.
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630.
- Lima, D.S.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Guilherme, M.R.; Scariot, D.B.; Nakamura, C.V.; Muniz, E.C.; Rubira, A.F. pH-responsive alginate-based hydrogels for protein delivery. J. Mol. Liq. 2018, 262, 29–36.
- Xu, H.L.; Xu, J.; Zhang, S.S.; Zhu, Q.Y.; Jin, B.H.; ZhuGe, D.L.; Shen, B.X.; Wu, X.Q.; Xiao, J.; Zhao, Y.Z. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017, 24, 867–881.
- Dutta, K.; Das, R.; Ling, J.; Monibas, R.M.; Carballo-Jane, E.; Kekec, A.; Feng, D.D.; Lin, S.; Mu, J.; Saklatvala, R.; et al. In Situ Forming Injectable Thermoresponsive Hydrogels for Controlled Delivery of Biomacromolecules. ACS Omega 2020, 5, 17531–17542.
- Hu, D.-N.; Ju, X.-J.; Pu, X.-Q.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.-Y. Injectable Temperature/Glucose Dual-Responsive Hydrogels for Controlled Release of Insulin. Ind. Eng. Chem. Res. 2021, 60, 8147–8158.
- Rahmanian, E.; Salari, N.; Mohammadi, M.; Jalali, R. Evaluation of sexual dysfunction and female sexual dysfunction indicators in women with type 2 diabetes: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 73.
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480.
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194.
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862.
