Human activities and population growth raise the energy demands for construction materials and produce a substantial volume of solid waste across diverse sectors like steel and iron production, mining, power production, agriculture, and the production of electronic goods [
1,
2,
3]. The handling and disposal of such waste streams have resulted in significant economic and environmental consequences. Hence, it is better to reuse or recycle some of the solid wastes into valuable resources, including construction materials, glass products, recycled energy, plastic products, and soil conditioners [
4]. Over the past few years, there has been a substantial development in research focused on using solid waste in precursors, aggregates, fibers, and more [
1]. Presently, alkali-activated materials (AAM), especially geopolymers (GPs), effectively make use of byproducts and industrial waste, often diverting them from improper disposal practices [
5]. Therefore, geopolymers are emerging as a promising alternative for producing sustainable construction materials. This approach not only aids in waste management but also contributes to developing environmentally friendly construction solutions [
6]. Ever since V. Glukhovsky’s initial discovery of alkali-activated binders in 1959 in Ukraine, extensive research has been dedicated to investigating and improving their physicochemical characteristics [
7]. In the late 1970s, Davidovits introduced the expression “geopolymer” to characterize the inorganic polymeric system created through the metakaolin alkali activation [
8]. Today, it stands as the most widely used term to refer to this material [
9]. As per Davidovits [
10], this innovative binder was produced through a modification of the techniques employed by the Romans and Egyptians. Davidovits goes as far as proposing that the pyramids might not have been constructed using natural stone but rather with man-made binders. His research suggests that the blocks of the pyramids were not composed of layers of calcium fossils, as is the case with natural stones. Instead, they were arranged randomly, much like in an artificial binder [
10]. From 1979 to 1995, Davidovits and his team extensively contributed to the field of geopolymerization with numerous published papers and granted patents. Notably, they pioneered the creation of a silico-aluminate mineral polymer, which shaped as a solid solution at temperatures reaching approximately 120 °C [
7]. Geopolymer is a groundbreaking aluminosilicate inorganic polymer characterized by an amorphous three-dimensional network structure consisting of silicon-oxygen and aluminum-oxygen tetrahedra interconnected through an oxygen bridge [
11]. The material, resulting from the geopolymerization of an alkali activator and active aluminosilicate precursor, boasts several benefits, including exceptional mechanical strength, improved durability, resistance to both acid and thermal influences, cost-effectiveness, and better environmental impacts, including reduced CO
2 emissions [
12,
13]. As depicted in
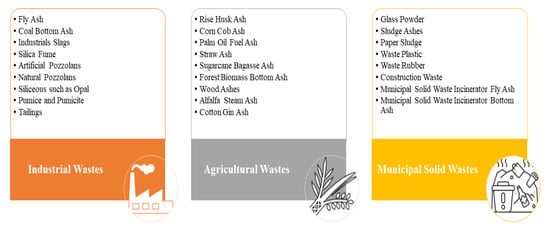
Figure 1, the solid wastes incorporated into geopolymers as potential aluminosilicate precursors can be primarily categorized into three main groups: agricultural wastes (AW), municipal solid wastes (MSW), and industrial wastes (IW) [
14]. Solid wastes containing aluminosilicate are often referred to as supplementary cementitious materials (SCMs). These materials have the potential to replace cement while maintaining similar effectiveness [
15]. Yet, measuring the performance of SCMs directly is a complex task. This complexity arises from the challenge of identifying how, or in which combination, these SCMs modify the properties of the cementitious materials, a process that indirectly reflects their Degree of Reaction (DOR) [
16]. The DOR of SCMs within hydrated Portland cement plays a significant role in formulating concrete with reduced carbon dioxide content. Numerous research efforts have been dedicated to finding ways to predict the DOR of SCMs in such a hydrated environment. A notable contribution in this field is by Degefa and colleagues, who developed a predictive model using a machine learning algorithm. This model, based on genetic programming and tailored for physical systems’ identification, paves the way for creating more environmentally sustainable and effective concrete designs, in line with current ecological goals [
16]. Furthermore, explorations into thermodynamic modeling effectiveness have been conducted to predict the DOR of SCMs in hydrant Portland cement. These studies have revealed that this approach can yield fairly accurate predictions regarding bound water content and the DOR of SCMs [
17]. Additionally, it has been observed that the reactivity of supplementary cementitious materials (SCMs) in cement mixtures remains active over an extended period. This ongoing reactivity plays a crucial role in enhancing the strength of the blend during prolonged hydration processes [
18]. Such observations underscore the need to factor in elements like the water-to-cement ratio, the duration of curing, the composition of oxides, and the use of thermodynamic modeling for accurately predicting the DOR of SCMs in hydrated Portland cement.
Implementing GP technology in the building industry provides the potential for improved environmental impact at the construction material level [
19]. Earlier studies have demonstrated that geopolymer concrete (GPC) surpasses conventional concrete (CC) in terms of environmental impact when employed as an alternative material [
20]. Coal fly ash (CFA)-based geopolymers are increasingly recognized as an eco-friendly substitute for conventional Portland cement in concrete [
21]. The process of geopolymerization presents an opportunity to replace cement with coal fly ash in construction, aiding in the pursuit of sustainable development [
22]. Incorporating coal fly ash into concrete significantly affects how water moves within the concrete’s structure. Research indicates that substituting cement binders with CFA reduces water absorption levels, especially when the replacement is up to 35% [
23]. Nevertheless, the impact of CFA on concrete’s characteristics is contingent on the proportion of CFA incorporated. Furthermore, Diatomaceous earth presents potential in the development of geopolymer concrete. It is characterized by its abundant silica content derived from fossilized algae [
24]. The reviewed studies suggest that incorporating diatomaceous earth can lead to the creation of eco-friendly, insulating, and lightweight construction materials, thereby reducing the detrimental environmental and economic impacts associated with industrial solid waste [
25]. Additionally, the research by Kipsanai et al. [
26] delved into the properties of geopolymer concrete made with alkaline-activated diatomaceous earth and reinforced with sisal fibers. The outcomes reveal that sisal-reinforced geopolymer concrete possesses impressive mechanical, physical, and durable characteristics, underscoring its viability as an eco-friendly and resilient material for construction.
2. Geopolymers Composition
A geopolymer is formed by connecting AlO
4− and SiO
4− tetrahedra, wherein each tetrahedron shares its corners with another tetrahedron through oxygen atoms, creating a 3D structure. This resulting structure is primarily amorphous, though it might contain a few zeolitic phases. The amorphous portion is referred to as N–A–S–H gel, named after the ultimate composition of the geopolymerization output (Na
2O−Al
2O
3−SiO
2−H
2O). Geopolymers are inorganic materials with polymeric structures produced by blending an alkaline solution with a dry solid, typically an aluminosilicate rich in Al and Si [
46].
For geopolymer solidification, the presence of aluminum (Al) is crucial. Mixtures containing elevated levels of alkali silicate concentration tend to be metastable since the silica tetrahedra are susceptible to water-attacks. This leads to the creation of silanol (Si–OH) pairs (Equation (1), leading eventually to the creation of Si(OH)
4 (Equation (2)). However, in an alkaline environment, Equation (2) dominates, causing the remaining oxygen bonds to weaken and the continued dissolution of the silicate. Hence, soluble silica alone is not sufficient for chemical hardening [
47].
The activating solution alkalinity leads to dissoluting aluminosilicates in the source material. In the process of molecular organization, some Si tetrahedra could be substituted with Al tetrahedra, resulting in negatively charged Al tetrahedra. These charges are balanced by positively charged alkaline cations from the activating solution [
48]. In the N–A–S–H gel, silicon primarily plays a role in the formation of zeolitic nuclei. It plays a crucial role in the early stages, especially with water glass (SS) as the activator, undergoing initial dissolution to supply monomers for silica-rich gel formation. However, excessive dimers in the silicate can lead to faster yet more metastable gel formation [
49]. Aluminum is actively involved in initial chemical reactions. A source rich in alumina releases more Al into the solution, enhancing source reactivity. An excess of Al can lead to reactions with crystalline products. Dissolved Al becomes part of the Si-rich gel structure, increasing its stability [
49]. Sodium acts as a charge balancer, stabilizing the gel by balancing Al monomers or filling pores in mixtures with zeolitic products [
49]. Al species transform to Al(iv) (or Al(OH)
4−) during source material activation, providing an indication of unreacted aluminosilicate. Crystalline phases, often zeolites, may form under specific conditions, increasing with higher alkalinity and lower soluble silica content. A high water content aids full hydration and minimizes interactions between ion pairs, allowing for unimpeded growth of gel precipitates [
50]. In summary, geopolymer formation depends on the interplay of Al and Si, with sodium balancing charges. The Si/Al ratio, aluminum content, and alkalinity affect the process, determining the characteristics of the resulting material. Additionally, factors like temperature and reaction time can lead to the formation of crystalline phases within the geopolymer structure.
3. Preparation of Geopolymer Concrete
Geopolymers are inorganic polymer materials created through the combination of various aluminosilicate source materials with alkali-activator solutions [
51]. Geopolymer cement concrete (GPCC) is a form of concrete produced with GP binder rather than ordinary Portland cement (OPC) [
34]. The main components of GPC are source materials, alkaline activators mixed with fine or coarse aggregates, and water [
52]. The aluminosilicate source materials may occur naturally, like metakaolin (MK), kaolin, bagasse ash, volcanic rock powder, and rice husk ash (RHA), or they might be produced industrially, like blast furnace slag (BFS), fly ash (FA), and silica fume (SF) [
53]. The primary alkaline activators (AAs) are sodium hydroxide (NaOH) and sodium silicate (Na
2SiO
3); nevertheless, any silicate or hydroxide can be employed as an activator, including potassium silicate (K
2SiO
3) and potassium hydroxide (KOH) [
52,
54]. GPCC might be one-part or two-part based on the activator source addition process. The one-part GPC, commonly referred to as “Just Add Water,” requires solely a dry mix along with water. This dry mix is made by combining a solid alkali activator accompanied by a solid aluminosilicate precursor, with the option of including or excluding the calcination process [
55]. Additionally, in the two-part GPCC, also known as conventional GPCC, the activators are introduced as a liquid state along with the water into a solid aluminosilicate precursor [
54].
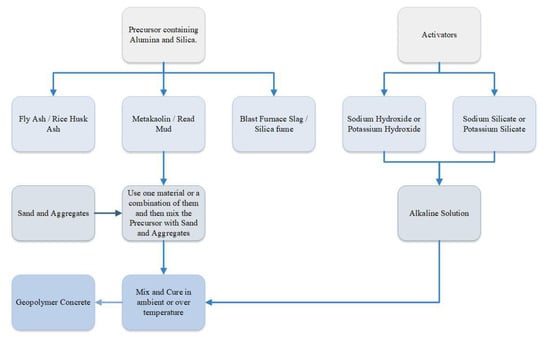
Figure 3 shows the flowchart for the process of creating a geopolymer concrete (GPC).
Figure 3. Flowchart for the process of creating a geopolymer cement concrete.
3.1. Aluminosilicate Precursors
GPCC uses aluminosilicates, which are industrial and natural byproducts containing amorphous silica and alumina. In underdeveloped nations, materials from industrial and agricultural waste, including amorphous silica and alumina, are frequently utilized for energy production. Repurposing these wastes in the manufacturing process of geopolymer technology and cement-based products offers a potential solution to the issue of ash disposal [
56].
3.1.1. Fly Ash (FA)
Coal fly ash is a commonly available anthropogenic material produced in thermal power plants as a byproduct of coal combustion [
57]. This industrial waste can create various environmental problems if released into the environment [
58]. FA composition varies greatly based on the burnt coal type, combustion process, conditions, and cooling control [
59]. However, it typically contains amounts of iron oxide (Fe
2O
3), calcium oxide (CaO), silicon dioxide (SiO
2), aluminum oxide (Al
2O
3), and other minor components [
60]. As per the guidelines presented by the American Society for Testing and Materials (ASTM) [
61], FA is divided into two primary types: C and F fly ash. Class F is originated from the combustion of either anthracite or bituminous coal. It has a pozzolanic nature, with CaO content of under 18%, while class C has pozzolanic and self-cementing properties, produced from sub-bituminous coal or the burning of lignite, and has more than 18% of CaO [
62]. Fly ash has several beneficial applications in multiple areas of the construction industry, such as the stabilization of soil, brick and block manufacturing, cement substitution in concrete, structural fill and embankment, constructing roads, asphalt pavement, and in dams [
63]. There are several trace elements found in coal that are extremely toxic to both people and other living things. The obtained fly ash after combusting the coal has higher concentrations of these elements; therefore, fly ash is thought to have a negative environmental impact if not appropriately managed [
64]. The correct management and utilization of fly ash may provide economic and environmental advantages while limiting harmful effects on the environment.
3.1.2. Blast Furnace Slag (BFS)
The production of iron in blast furnaces results in the generation of blast furnace slag (BFS) as a byproduct. Iron ore, coke, and limestone are used to feed the furnaces. During the procedure, iron ore is converted into iron, and the remaining components combine to produce the slag. The created slag is then extracted as a molten liquid and allowed to cool [
65]. There are two primary forms of BFS: granulated blast furnace slag (GBFS) and air-cooled blast furnace slag (ABFS). GBFS is made by quickly cooling molten slag with water or steam, which creates a glassy, granular material. ABFS is formed by enabling molten slag to cool slowly in the open air, which leads to a denser, more crystalline substance [
66,
67]. BFS is used as a substitute for cement, reducing the amount of clinker required for cement production. BFS cannot substitute cement completely; however, partial cement replacement gives good results and a greener approach in the construction field [
68]. Additionally, BFS can serve as a suitable material for the production of geopolymer due to its high alumina and silica content [
69], which might be considered a solution for industrial waste and a promising approach for developing sustainable materials.
3.1.3. Silica Fume (SF)
Silica fume (SF), which is additionally called microsilica, is a valuable byproduct derived by an electric-arc furnace (EAF) throughout the manufacturing of silicon (Si) and ferrosilicon (FeSi) alloys [
70]. SF comprises of very small particles, each with an average diameter of 0.1 µm [
71]. This extremely small size of silica fume particles enables them to fill the voids that would otherwise remain unfilled. This characteristic results in a denser microstructure, contributing to elevated strengths, enhanced durability, and reduced permeability in materials [
70]. Moreover, SF is a highly reactive pozzolan due to its chemical, mineralogical, and physical properties, which may be derived from natural or artificial sources. Additionally, whether with low or high silica content, SF possesses a nano-porous formation, serving as a valuable supplementary substance for GP within concrete applications [
71]. The study carried out by Okoye et al. revealed that introducing silica fume produces an improvement in the compressive strength of the GPC produced. Additionally, increased flexural and tensile strengths were observed with escalating levels of SF content [
72]. Hence, adopting silica fume fulfills a crucial role in enhancing the characteristics of the geopolymers, paving the way for more sustainable and innovative alternatives to traditional cement-based materials.
3.1.4. Metakaolin (MK)
Metakaolin (MK) is an essential material used for producing geopolymers. It is mainly a pozzolanic material created from kaolin (China clay) clay through calcination at high temperatures (600 to 900 °C), where it undergoes amorphization and develops into a material with high reactivity [
73]. Examinations of durability indicate that metakaolin geopolymers have enhanced characteristics with regard to water resistance, thermal resistance, and resistance against corrosion. [
74]. The metakaolin-based geopolymers displayed enhanced workability in comparison to OPC as the proportion of sodium silicate (Na
2SiO
3) to sodium hydroxide (NaOH) was elevated up to a particular level. Beyond that level, workability decreased due to the elevated mixture viscosity [
75,
76]. The MK-based geopolymers’ mechanical characteristics were examined using orthogonal tests by Dai et al. The findings revealed that sodium- and potassium-based geopolymers displayed enhanced compressive and flexural strength compared to other binding materials [
77]. Metakaolin serves as a primary product; thereby, its production remains unaffected by market fluctuations in other sectors. Nonetheless, despite its global availability, the current metakaolin production volume remains insufficient to adequately satisfy the global need for pozzolanic materials in the production of cement and concrete [
70].
3.1.5. Rice Husk Ash (RHA)
The rice husk ash (RHA) is a byproduct obtained by cultivating and processing rice. About 20–25% of the rice paddy comprises of an outer husk that cannot be digested. This husk is often separated and burned in nearby power facilities to provide steam for parboiling rice, in domestic stoves, or as fuel for producing electricity. Burning these husks turns them into ash, constituting roughly 18% of their original weight. Consequently, the production of one ton of rice yields around 45 kg (70 lb) of rice husk ash [
70,
78,
79]. This ash contains a high silica content (between 80% and 95%) and distinctive pozzolanic properties [
80]. Concrete containing rice husk ash (RHA) could extend the setting time of the cementitious paste while also improving the workability of the concrete mixture in comparison to OPC. Additionally, GPC made with RHA has the capacity to minimize the permeability and overall porosity of the concrete [
71]. The utilization of RHA in geopolymers has gained remarkable interest recently due to its potential to enhance the mechanical characteristics, sustainability, durability, and cut some of the production costs compared to OPC [
81].
3.1.6. Red Mud (RM)
Red mud (RM) is a byproduct material produced during the extraction of alumina from bauxite via Bayer’s process [
82]. Between roughly 1 and 2.5 tons of RM are generated for every 1 ton of alumina extracted, accounting for approximately 55% to 65% of the processed bauxite [
83]. RM possesses distinctive properties, including a high pH level ranging from 10 to 12.5 [
52], a substantial solids content spanning between 15% and 30%, and a varying chemical composition. Notably, its red color arises from its iron oxide content (Fe
2O
3), which varies between 20% and 60%. The additional components include aluminum oxide (AI
2O
3) at 10–30%, silicon dioxide (SiO
2) at 2–20%, sodium oxide (Na
2O) at 2–10%, calcium oxide (CaO) at 2–8%, as well as trace amounts of titanium dioxide and additional oxides, collectively reaching up to 28% [
55]. Researchers have been actively exploring ways to employ red mud into both OPC manufacturing and alkali-activated binder formulations [
84]. Incorporating a small percentage of red mud as a partial substitution for cement in GPC has been demonstrated to significantly enhance key mechanical properties, including Young’s modulus (E), compressive strength (CS), and failure strain in the resulting material [
71].
Table 1 displays the chemical composition of frequently employed precursors in GP manufacturing. Notably, there is a noticeable variation in the chemical composition across different precursor types.
Table 1. The chemical composition of RHA, BFS, RD, MK, FA, and SF (wt, %).
3.2. Activator
In GPC, alkaline activators, including liquid and solid, are generally employed to polymerize aluminosilicates. Alkaline activators (Aas) are used to initiate the polymerization of aluminosilicates to produce geopolymer GPC. The alkaline solutions utilized are often powerful and can contain compounds such as potassium hydroxide (KOH), sodium hydroxide (NaOH), potassium silicate (K
2SiO
3), sodium silicate (Na
2SiO
3), or a mix of these silicates and hydroxides. These activators dissolve the silicon (Si) and aluminum (Al) atoms, allowing them to recombine into the geopolymeric network [
54,
94]. The type of activators can impact the geopolymer material’s microstructure and mechanical properties, including durability, setting time, and strength [
95]. Prior research has indicated that sodium-based alkali activators tend to exhibit greater activation efficiency in comparison to potassium-based activators for F fly ash [
96]. Nevertheless, another study [
97] discovered that the incorporation of potassium compounds in geopolymer systems resulted in heightened alkalinity compared to the utilization of NaOH. The majority of geopolymers are activated through the utilization of alkali activators; however, others are activated using acidic activators [
98]. Acid-based activators offer a compelling alternative to the more commonly favored alkaline option. These activators are typically derived from either phosphate-based acids or humic-based acids [
99]. Humic acids, as natural organic acids, are not commonly employed in this field of study due to the complex nature of their composition. Consequently, the application of acidic activation has predominantly centered on phosphate-based activators [
99]. The most popular phosphate-based activator is phosphoric acid (PA). Furthermore, another type of phosphate-based activator in the field of geopolymers production is aluminum phosphate based activators, like Al(H
2PO
4)
3, AlH
3(PO
4)
2•3H
2O, and Al(HPO
4)
3 [
100].