Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Antimicrobial resistance is a severe and developing clinical issue that has reduced the therapeutic effectiveness of conventional antibiotics and narrowed the treatment choices for bacterial infections. Antibiotic-resistant bacteria are generally difficult to treat due to reduced membrane penetration, efflux pump overexpression, target site shifting, inactive subpopulations, biofilm growth, and enzymatic destruction. Resistant bacteria are strains resistant to several medicines, resulting in increased infections.
- Latin American plants
- microorganisms
- bacteria
- fungi
- virus
1. Introduction
Infectious diseases are a significant source of public health issues. Despite breakthroughs in creating and manufacturing antivirals and antibiotics, bacteria, viruses, and other microorganisms continue to kill millions of people each year.
Antimicrobial resistance is a severe and developing clinical issue that has reduced the therapeutic effectiveness of conventional antibiotics and narrowed the treatment choices for bacterial infections. Antibiotic-resistant bacteria are generally difficult to treat due to reduced membrane penetration, efflux pump overexpression, target site shifting, inactive subpopulations, biofilm growth, and enzymatic destruction. Resistant bacteria are strains resistant to several medicines, resulting in increased infections [1].
Many bacteria may infect and live in their hosts for extended periods. This might be related to host immunosuppression, pathogen immune evasion, and/or inadequate drug clearance. Bacteria that are resistant or tolerant to antibiotics can survive treatment. Persistent bacteria are a transiently antibiotic-tolerant subset of bacterial cells that grow slowly or cease developing but can resume proliferation after exposure to fatal stress. Persistent cell production creates phenotypic variation within a bacterial population, significantly enhancing the odds of effectively responding to environmental change. The existence of resistant cells can lead to the emergence and recurrence of chronic bacterial infections and an increased risk of antibiotic resistance [2].
Emerging viral infections, on the other hand, continue to be a severe concern for worldwide public health. In 1997, it was revealed that a highly virulent avian influenza A (H5N1) virus may be transferred directly from poultry to people, in contrast to previously known human-to-human and livestock-to-human modes of transmission, raising severe fears about a probable influenza pandemic. Several additional avian influenza A virus subtypes (H7N9, H9N2, and H7N3) have also been linked to human sickness, increasing concerns that all influenza A virus subtypes circulating in domestic poultry and cattle in the wild might transmit to people and cause pandemics.
The most recent viral pneumonia epidemic, which began in mid-December 2019 (COVID-19) in Wuhan, China, and has spread swiftly throughout the world, is a stark reminder of the vulnerability to new viral illnesses. Tens of thousands of people are currently infected with SARS-CoV-2 [3].
In the case of fungus, it is believed that roughly 5 million species are extensively ubiquitous in the environment, of which approximately 300 can cause infections in people. However, only 20–25 are commonly seen in the clinic and are the cause of sick patients. Patients with HIV, organ transplant recipients, or those undergoing chemotherapy are examples of such people. The most frequent fungal diseases are Candida spp., Cryptococcus spp., Aspergillus spp., and Pneumocystis spp., which cause around 2 million illnesses and 1 million deaths yearly [4].
2. Plant Antimicrobials
2.1. Antimicrobial Resistance
As COVID-19 rages, the antimicrobial resistance (AMR) epidemic continues in the background. AMR causes recurrent microbe (viruses, bacteria, and fungi) infections that lengthen hospital stays and result in preventable deaths. It is estimated that 4.95 million people died due to AMR in 2019 and that by 2050, there will be 10 million annual deaths due to antimicrobial resistance. Two factors primarily cause antimicrobial resistance. The first is the overuse of antimicrobials, which exposes microbes to them regularly, increasing their chances of developing resistance. The second issue is that few new antimicrobial drugs are being developed to replace ineffective ones due to rising drug resistance [5][6].
Compared to non-resistant forms, resistant bacteria are two times more likely to develop into a serious health problem and are three times more likely to lead to death [7][8]. Resistance to first-line antibiotics, such as fluoroquinolones and lactam antibiotics, is responsible for more than two-thirds of AMR-related deaths (carbapenems, cephalosporins, and penicillins). People with low incomes are disproportionately affected by AMR because they have limited access to more expensive second-line antibiotics that may be effective when first-line drugs fail. Physicians should avoid inappropriate antibiotic therapy when, for example, the illness has a viral origin [5][9][10].
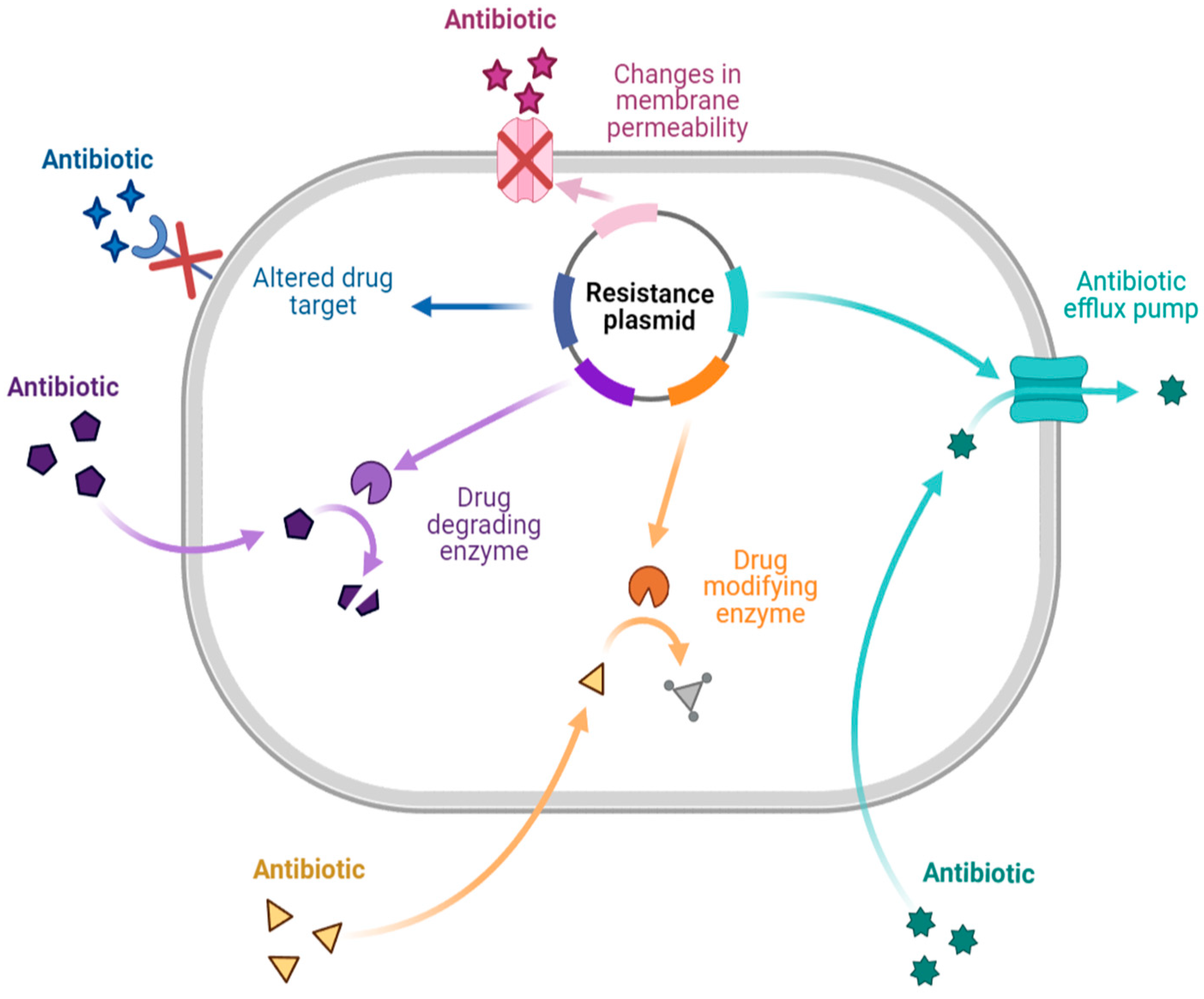
There are different mechanisms of resistance to antibiotics (Figure 1). Bacteria produce enzymes that can destroy or alter the structure of the drug, causing the drug to lose its activity during enzymatic inactivation. Drug-inactivating enzymes are classified into three types: hydrolase (primarily lactamase), passivating enzymes (aminoglycoside-inactivating enzyme, chloramphenicol acetyltransferase, and erythromycin esterase), and modifying enzymes (aminoglycoside-modifying enzyme). Similarly, changing the target to which the drug is directed ensures that the antibiotic binds appropriately to the bacteria. This mechanism is primarily seen in Gram-positive bacteria with drug resistance and polymyxin resistance. Changes in outer membrane permeability that result in channel alteration or decreased expression make the bacteria less sensitive. In the drug efflux pump, when the drug is removed from the bacterial cytoplasm, the concentration is much lower than is required for it to exhibit activity, resulting in drug resistance. This process requires energy and works with various antibiotics [6][11][12][13].
2.2. Natural Products and Plant Extracts with Antimicrobial Activity against MDR Strains
Multidrug resistance (MDR) is a major cause of human suffering because it undermines doctor–patient trust, resulting in massive economic losses. In this world of microbe–man cohabitation, the survival of the human species will be compromised in the absence of health-giving microbes, and there will be no way to avoid the emergence of MDR superbugs. Throughout history, the isolation and identification of biologically active compounds and molecules from nature have resulted in the discovery of new therapeutics, advancing the health and pharmaceutical industries. Phytochemicals are used in the research and development of the pharmaceutical industry as a source of new molecules, leading to the development of novel drugs [14][15].
As shown in Table 1, several classes and subclasses of secondary metabolites (Figure 2) have been isolated from plants with antimicrobial activity, each with a different mechanism of action. This table shows that, depending on the compound class, they share the same kind of mechanism of action.
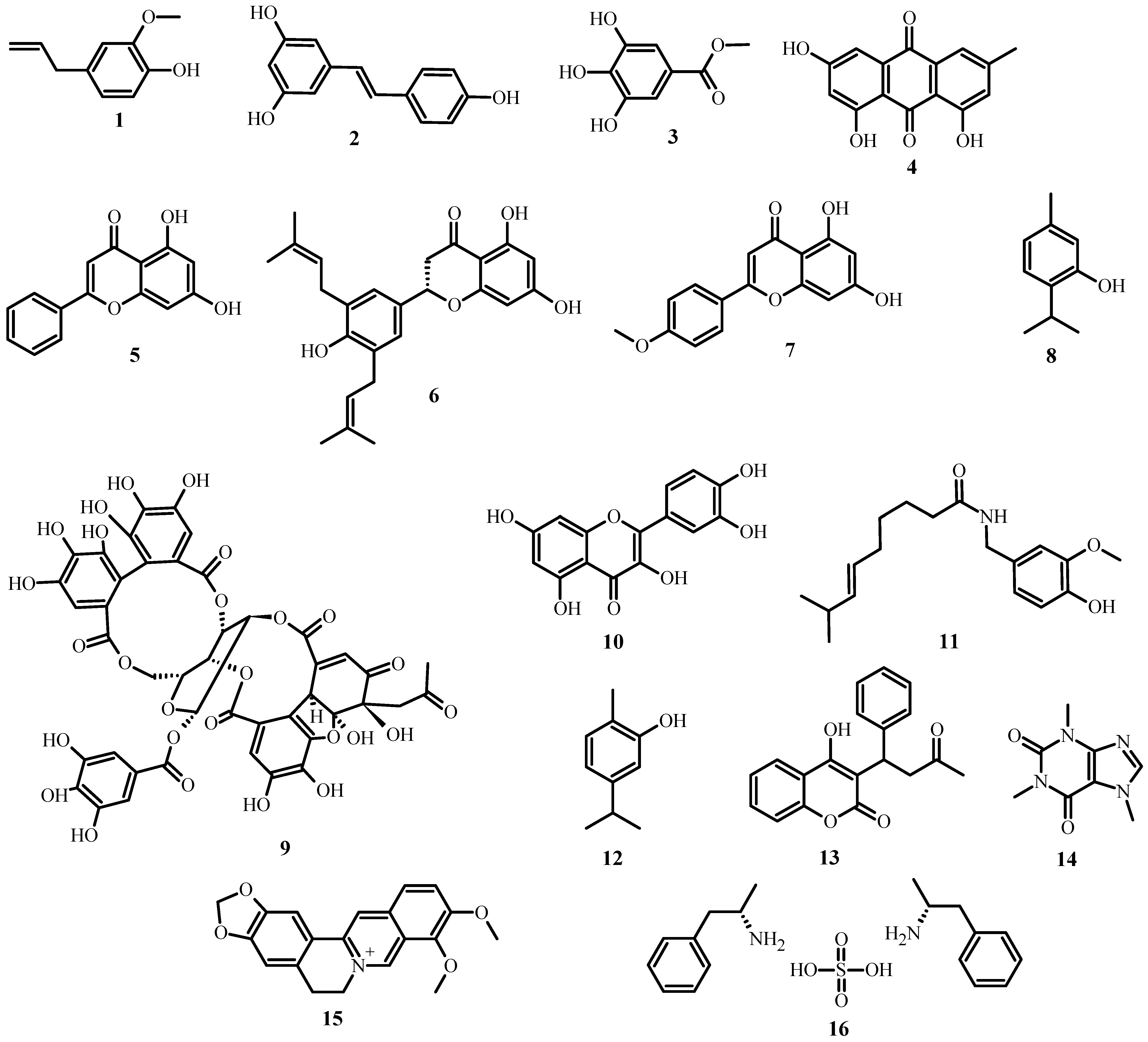
Figure 2. Structures of plant antimicrobial compounds found in medicinal plants from Latin America, from Table 1.
Regarding essential oils, the essential oil of rosemary (Rosmarinus officinalis) was found to have antibacterial activity against three types of MDR acne-causing bacteria: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes [16]. Similarly, volatile oils extracted from cinnamon (Cinnamomum verum) and tree basil (Ocimum gratissimum) had potent bactericidal activity against MDR A. baumannii bacteria [17].
Terminalia bellirica fruits were studied, and it was discovered that the aqueous and methanol extracts had antibacterial activity against all strains of MRSA (Methicillin-resistant Staphylococcus aureus), MDR Acinetobacter spp., and MDR P. aeruginosa [18].
The aqueous, hexane, and ethanol extracts of Punica granatum peel demonstrated antibacterial activity against MDR pathogens such as P. aeruginosa and A. baumannii. Valoneic acid dilactone (aqueous fractions), Hexoside (ethanol fractions), and Coumaric acid (hexane fractions) were discovered to be bioactive compounds [17]. Ethanolic extracts of Azadirachta indica, Allium sativum, and Syzygium cumini were found to have anti-MDR-Candida spp. activity. According to a phytochemical analysis of ethanolic plant extracts, all the plants studied contained alkaloids, flavonoids, glycosides, phenols, tannins, and saponins [19].
ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) MDR pathogens were tested using various extracts. Three ethanolic extracts from Adiantum capillus-veneris, Artemisia absinthium, and Martynia annua were found to inhibit the growth of MDR strains of ESKAPE pathogens [20].
Aside from the plant extracts mentioned above, various plant compounds (Figure 3) with anti-MDR bacteria activity have already been identified. Table 2 lists these compounds, as well as the biological effects they have on specific strains.
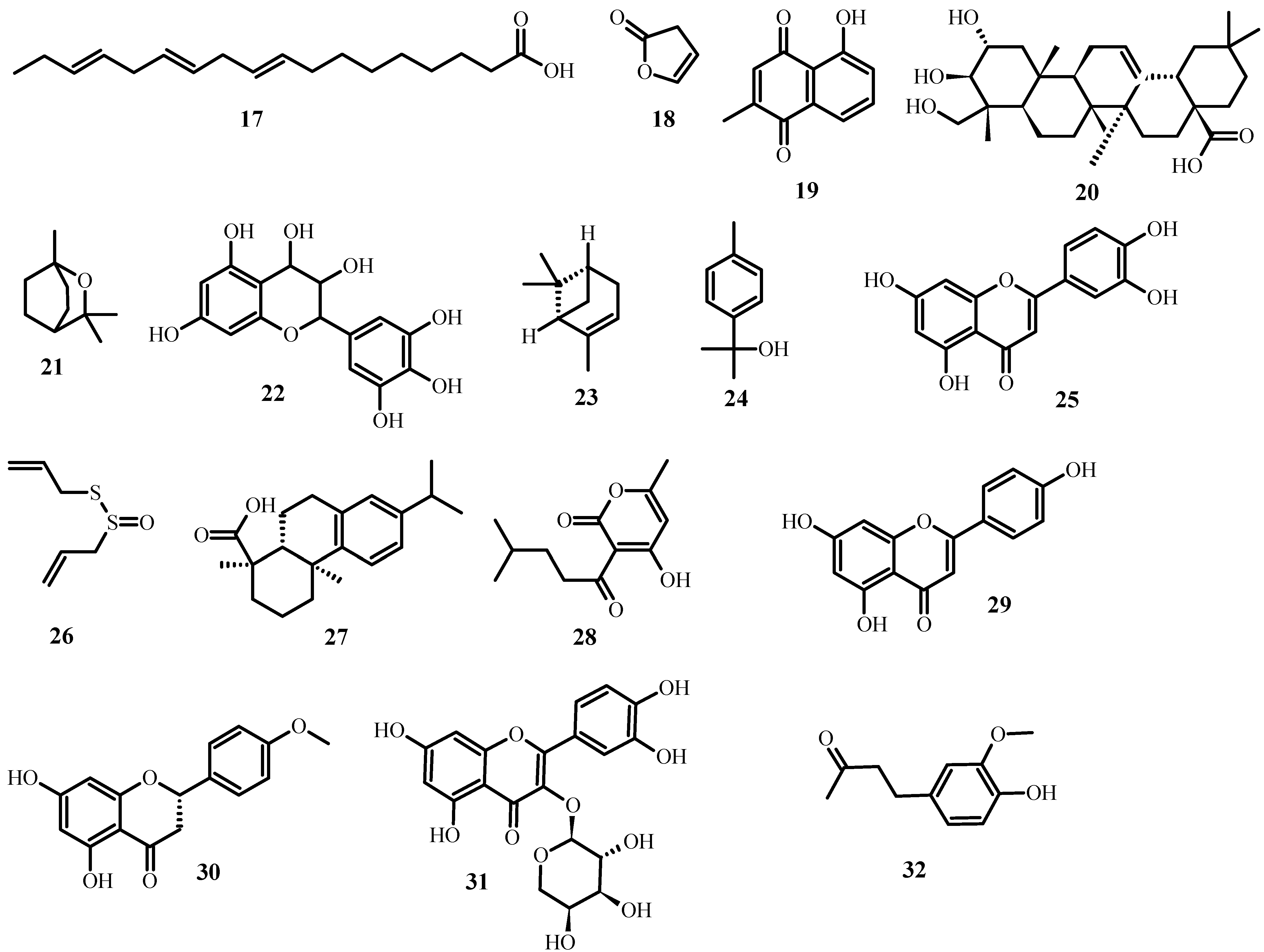
Figure 3. Structures of promising plant-derived compounds merit combating drug-resistant pathogenic microbes, from Table 2.
Because of the severe problem of MDR properties in microbes, the discovery of alternative drugs from natural products should be one of the primary goals of current research. Understanding the nature of pathogenic microbes, recognizing biofilm formation and architectural scheme, and employing cross-disciplinary techniques are thus critical for discovering new potent and novel drugs.
Table 1. Antimicrobial mechanisms of plant compounds present in Latin American medicinal plants.
| Class | Subclass | Examples | Source of the Compound | Mechanism | References |
|---|---|---|---|---|---|
| Phenolics | Simple phenols | Eugenol (1) | Syzygium aromaticum | Membrane disruption. | [21][22] |
| Resveratrol (2) | Vitis vinifera | Binds reversibly to ATP synthase. | [21][23] | ||
| Phenolic acids | Methyl gallate (3) | Euphorbia hyssopifolia | Inhibits DNA gyrase or ATPase. | [21][24] | |
| Quinones | Emodin (4) | Rheum rhabarbarum | Destroys the integrity of the cell wall and cell membrane. | [21][25] | |
| Flavonoids | Chrysin (5) | Passiflora caerulea | Binds to adhesins. | [26][27] | |
| Flavones | Abyssinone V (6) | Erythrina abyssinica | Complexes with the cell wall, inactivate enzymes and inhibit HIV reverse transcriptase. | [26] | |
| Acacetin (7) | Robinia pseudoacacia | - | [21] | ||
| Flavonols | Quercetin (10) | Brickellia cavanillesii | Disrupts bacterial cell walls and cell membranes, disrupt nucleic acid synthesis, inhibit biofilm formation, and reduce expression of virulence factors. | [27][28] | |
| Tannins | Ellagitannin (9) | Punica granatum | Binds to proteins, bind to adhesins, enzyme inhibition, substrate deprivation, complex with the cell wall, membrane disruption, metal ion complexation. | [26] | |
| Coumarins | Warfarin (13) | Melilotus officinalis | Interacts with eukaryotic DNA (antiviral activity). | [26] | |
| Terpenoids | Capsaicin (11) | Capsicum annuum | Membrane disruption. | [26] | |
| Carvacrol (12) | Xylopia aromatica | Membrane disruption. | [21][29] | ||
| Thymol (8) | Induces the permeability and depolarization of the cytoplasmic membrane. | [21][30] | |||
| Alkaloids | Caffeine (14) | Coffea arabica | Inhibits biofilm development. | [21][31] | |
| Berberine (15) | Argemone mexicana | Damages bacterial cells by destroying cellular proteins. | [21][32] | ||
| Lectins and polypeptides | Fabatin (16) | Vicia faba | Blocks viral fusion or adsorption and forms disulfide bridges. | [26] |
Table 2. Different compounds derived from plants with promising activity to combat drug-resistant pathogens (based on [33]).
| Name of the Compound | Source of the Compound | Biological Effect on MDR Bacteria | References |
|---|---|---|---|
| 9,12,15-Octadecatrienoic acid (17) | Ocimum basilicum | Used in contesting E. coli, S. aureus, K. pneumonia, P. aeruginosa, and P. mirabilis. | [33] |
| Furanone (18) | Vanilla planifolia | Interferes in the quorum sensing system of P. aeruginosa. | [34] |
| Plumbagin (19) | Plumbago indica | Has antibacterial properties by binding to the ATP cassette transporter. | [35][36] |
| Arjunolic acid (20) | Cercidium microphyllum | Inhibits E. coli, B. subtilis, and S. sonnei. | [37] |
| 1,8-Cineole (21) | Eucalyptus globulus | Has antibacterial (methicillin-resistant S. aureus), antibiofilm, and anti-quorum sensing activities. | [38][39] |
| Leucoanthocyanidin (22) | Umbellularia californica | Has a cidal effect against B. cereus ATCC14579, S. pyogens ATCC10782, and MRSA ATCC-BAA-1683. | [40] |
| Quercetin (10) | Citrus sinensis | Inhibits the proton motive force (PMF) of S. aureus and inhibits P. aeruginosa (POA1), E. coli O157H7, and V. harveyi BB120. | [41] |
| Warfarin (13) | Dipteryx odorata | Inhibits S. viridans, S. mutans and S. aureus. | [15] |
| α-Pinene (23) | Callistemon viminalis | Suppresses the growth of B. cereus, S. typhi, P. aeruginosa, B. subtilis, E. coli, and P. vulgaris. | [42] |
| p-Cymen-8-ol (24) | Senecio nutans | Interferes with the membrane permeability of V. cholerae. | [43] |
| Luteolin (25) | Guazuma ulmifolia | Has a cidal effect against M. tuberculosis. | [44] |
| Allicin (26) | Allium sativum | Interferes with the metabolic systems of H. pylori, S. epidermidis, B. cepacia, P. aeruginosa, and S. aureus. | [45] |
| Thymol (8) | Lippia sidoides | Has activity against L. monocytogen, S. typhimurium, and E. coli O157:H7. | [45][46] |
| Dehydroabietic acid (27) | Pinus elliottii | Has a cidal effect against E. faecalis, S. haemolyticus, S. capitis, and MDR-S. epidermidis. | [47] |
| Pogostone (28) | Pogostemon cablin | Is effective against both gram-negative and gram-positive bacteria. | [48] |
| Apigenin (29) | Mentha pulegium | Interferes with the growth of B. cereus, E. coli, and S. aureus. | [49] |
| Isosakuranetin (30) | Hyptis albida | Inhibits S. aureus and B. subitilis. | [50] |
| Guaijaverin (31) | Psidium guajava | Significantly inhibits the adherence of S. mutans. | [51][52] |
| Zingerone (32) | Zingiber officinale | Inhibits biofilm formation and attenuation of motility properties in P. aeruginosa. | [53][54][55] |
2.3. Pathogenic Fungi for Human
Fungi are eukaryotic organisms widely distributed across the planet, with more than 700,000 species classified [56]; however, it is estimated that there may be more than 1 million species in existence [57]. Despite these data, the number of fungi that can affect other species is minimal, with less than 0.1% being of medical importance to humans, and less than 50 species being identified as pathogenic fungi. In recent years, fungi adapted to modified ecosystems have significantly impacted human health, as they tend to infect plants and their metabolism, negatively affecting the food web [58][59].
Mycoses are usually superficial, cutaneous, systemic, or opportunistic. A worldwide risk factor is immunosuppression; however, the microbiome imbalance caused by antibiotics must be considered, as it can lead to an even more severe infection [60][61]. It is widely thought that most mycoses are opportunistic. It is extremely important to take into account that mycosis can be considered dangerous due to the entry of several fungi, with cosmopolitan genera such as Candida, Cryptococcus, and Aspergillus being prevalent [62][63][64], while creating invasive fungal infections (IFIs) that cause high mortality rates worldwide [59][65][66].
2.4. Mechanism of Action and Drug-Resistance of Pathogenic Fungi
Pathogenic fungi create complex signaling cascades that depend on the host and environment [67]. A 2017 review points out the importance of recognizing the pathways involved in fungal pathogenicity and identifying opportunity areas to create better antibiotics [68], even if knowing these factors would make it impossible to create efficient vaccines [69][70]. However, current antifungal drugs have different mechanisms of action (Table 3); the most common mechanisms are directed against the fungal cell wall or membrane, specifically against ergosterol or (1,3)-β-d-glucan biosynthesis, except for pyrimidines and orotomides that target crucial molecules in nucleic acid metabolism [71][72][73][74].
Table 3. Mechanisms of action of families of antifungal drugs.
| Family of Antifungal Drugs | Mechanism of Action | References |
|---|---|---|
| Azoles (fluconazole) |
Inhibit fungal cell cytochrome P-450-3-A, disrupting ergosterol synthesis and intoxicating the cell with sterol intermediates. | [71][72][73] |
| Polyene (anfotericine B) |
Binds to ergosterol and generates pores in the membrane, causing oxidative damage and cell death. | [71][72] |
| Echinocandins (micafungin) |
Inhibit the enzyme 1,3-β-D-glucan synthase, which weakens the cell wall, causing osmotic instability. | [71][72] |
| Allylamines (terbinafine) |
Block the enzyme squalene epoxidase, reducing ergosterol levels and increasing squalene. This increases the permeability of the cell. membrane, causing a decrease in fungal growth. | [71] |
| Pyrimidines (flucytosine) |
Bind to cytosine permease, already in the nucleus, and generate fluorardilic acid, which is incorporated into the RNA, rendering it useless. | [71][73] |
| Orotomides (olorofim) |
Inhibit dihydroorotate dehydrogenase synthesis, preventing the synthesis of DNA and RNA. | [75][76] |
| Fosmanogepix | Inhibits the enzyme Gwt1, responsible for glycosylphosphatidylinositol synthesis. | [74] |
Just as bacteria generate drug resistance, so do fungi; this drug resistance can be described from a clinical point of view, referring to the worsening of an infection despite receiving adequate drug treatment. On the other hand, in the laboratory context, resistance is evaluated through a Minimum Inhibitory Concentration (MIC) assay to determine the growth of the pathogen at different concentrations of antibiotics [67][68][77]. It is necessary to point out the concept of drug tolerance, which is considered as the fungus persistence on the substrate; however, its growth is slow due to multifactorial causes [78][79].
2.5. Latin American Plants with Antifungal Effects
Fungi drug resistance has created a worldwide clinical challenge, and treatment alternatives have been considered, such as including two or more antifungals for one treatment; however, this does not make a significant difference [80]. This is why alternatives should be considered, such as using plant-derived compounds that can act via bypassing common metabolic pathways in fungal pathology. Table 4 summarizes the medicinal plant extracts with antifungal properties.
Table 4. Extracts of Latin American plants with activity against pathogenic fungi.
| Species | Extract | Fungi | References |
|---|---|---|---|
| Achyrocline satureioides | Ethanolic | Fusarium verticillioides | [81] |
| Achyrocline tomentosa | Ethanolic | Fusarium verticillioides | [81] |
| Aloysia citriodora | Ethanolic | Fusarium verticillioides | [81] |
| Annona cherimola | Ethanolic | Fusarium oxysporum | [82] |
| Annona muricata L. | Ethanolic | Candida albicans | [83] |
| Aristolochia argentina Griseb. | Ethanolic | Fusarium verticillioides | [81][84] |
| Asclepias curassavica | Hexanic, Methanolic | Candida albicans | [85] |
| Baccharis artemisioides | Ethanolic | Fusarium verticillioides | [81] |
| Baccharis flabellata | Ethanolic | Fusarium verticillioides | [81] |
| Baccharis salicifolia | Ethanolic | Fusarium verticillioides | [81] |
| Bixa orellana | Ethanolic | Candida albicans | [86] |
| Curcuma zedoaria | Acetone, Hexanic | Candida albicans | [87][88] |
| Dalea elegans | Ethanolic | Fusarium verticillioides | [81] |
| Echinacea angustifolia | Ethanolic | Cryptococcus neoformans | [89] |
| Echinacea atrorubens | Ethanolic | Cryptococcus neoformans | [90] |
| Echinacea pallida | Ethanolic | Candida albicans | [90] |
| Echinacea purpurea | Ethanolic | Saccharomyces cerevisiae | [89] |
| Eupatorium buniifolium | Methanolic | Trichophyton mentagrophytes | [91] |
| Euphorbia hyssopifolia | Methanolic | Aspergillus niger | [92] |
| Flourensia oolepis | Ethanolic | Fusarium verticillioides | [81] |
| Gaillardia megapotamica | Ethanolic | Fusarium verticillioides | [81] |
| Galphimia glauca | Hexanic, Methanolic | Trichophyton mentagrophytes | [85][93] |
| Grindelia pulchella | Ethanolic | Fusarium verticillioides | [81] |
| Heterothalamus alienus | Ethanolic | Fusarium verticillioides | [81] |
| Hibiscus sabdariffa | Methanolic | Candida albicans | [94] |
| Kageneckia lanceolata | Ethanolic | Fusarium verticillioides | [81] |
| Larrea cuneifolia | Ethanolic | Lenzites elegans | [95] |
| Larrea divaricata | Ethanolic | Penicillium notatum; Candida spp. | [95][96] |
| Lepechinia floribunda | Ethanolic | Fusarium verticillioides | [81] |
| Lippia turbinata | Ethanolic | Fusarium verticillioides | [81] |
| Loeselia mexicana | Ethanolic | Trichophyton mentagrophytes | [97] |
| Lygodium venustum | Ethanolic | Candida albicans | [98] |
| Lysiloma acapulcensis | Hexanic | Trichophyton mentagrophytes | [99] |
| Miconia mexicana | Methanolic | Candida albicans | [99] |
| Microliabum candidum | Ethanolic | Fusarium verticillioides | [81] |
| Minthostachys verticillata | Ethanolic | Fusarium verticillioides | [81] |
| Morrenia brachystephana | Ethanolic | Fusarium verticillioides | [81] |
| Otholobium higuerilla | Ethanolic | Fusarium verticillioides | [81] |
| Passiflora caerulea | Methanolic | Aspergillus flavus | [100] |
| Pimenta dioica | Essential oil | Fusarium oxysporum | [101] |
| Polygonum acuminatum | Dichloromethane | Cryptococcus neoformans | [102] |
| Salix alba | Methanolic | Aspergillus ornatus | [103] |
| Salvia cuspidata | Ethanolic | Fusarium verticillioides | [81] |
| Sebastiania commersoniana | Ethanolic | Candida spp. | [104] |
| Senecio vira-vira | Ethanolic | Fusarium verticillioides | [81] |
| Smilax domingensis | Ethanolic | Candida albicans | [105] |
| Syzygium aromaticum | Essential oil | Candida spp. | [106] |
| Terminalia triflora | Methanolic | Trichophyton mentagrophytes | [91] |
| Thalictrum decipiens | Ethanolic | Fusarium verticillioides | [81] |
| Tithonia diversifolia | Aquous | Fusarium oxysporum | [107] |
| Trichocline reptans | Ethanolic | Fusarium verticillioides | [81] |
| Vernonia mollisima | Ethanolic | Fusarium verticillioides | [81] |
| Vernonia nudiflora | Ethanolic | Fusarium verticillioides | [81] |
| Vitis vinifera | Aqueous | Candida spp. | [108] |
| Zanthoxylum coco | Ethanolic | Fusarium verticillioides | [81] |
| Zinnia peruviana | Ethanolic | Fusarium oxysporum | [95] |
| Zuccagnia punctata | Ethanolic | Aspergillus niger | [95] |
| Zuccagnia punctata | Dichloromethane | Candida albicans | [109] |
2.6. Medicinal Plant Antiviral Activity against Human-Infecting Viruses
In 2018, over 4400 virus species were classified into 122 families and 7535 [110] subfamilies. Human-infecting viruses include RNA viruses, DNA viruses, retroviruses, bare viruses, and virions, with RNA viruses being the most prevalent. Numerous medicinal plants contain compounds that inhibit the replication of viruses or enhance the immune system. Alkaloids, terpenes, flavonoids, numerous glucosides, and proteins have been recognized as phytochemicals; their metabolites include apigenin (29), kaempferol (34), and luteolin (25), in addition to the triterpenoids oleanolic acid (35) and ursolic acid (36) [111].
2.6.1. Biological Mechanisms of Antiviral Activity
Plant biocompounds may function similarly to conventional antiviral medications by inhibiting viral replication polymerase, protease, integrase, fusion molecules, and cell membrane binding. For example, the exposure of non-enveloped norovirus to 0.5% of carvacrol (12) results in the degradation of its capsid [112]. Some polysaccharides may also deter viruses from attaching to cells, while thiophenes, terpenoids, and polyacetylenes can interact with the membrane of infected cells [113]. Lignans, phenolic compounds, terpenoids, flavonoids, alkaloids, and furocoumarins can all inhibit viral replication. Biocompounds of Allium sativum are among the most studied; they have antiviral activity against human, animal, and plant infections. Multiple of these metabolites can strengthen the immune system’s response to infections. This biocompound interacts in vivo with thiols such as glutathione and L-cysteine to produce S-allyl-mercapto-glutathione (SAMG) and S-allyl-mercapto-cysteine (SAMC), which can degrade viral protein. In addition, it contains lectins, flavonoids (kaempferol (34), quercetin (10), and myricetin (37)), polysaccharides (fructan), steroids, saponins, fatty acids (lauric (38) and linoleic acid (39)), diverse enzymes, vitamins (A, B1, and C), allixin (40), minerals (Ca, Cu, Fe, K, Mg, Zn, and Se), and amino acids [114].
Table 5 summarizes the medicinal plant extracts and their possible mechanisms of action (Figure 4), while Table 6 discusses the medicinal plant biocompounds with antiviral properties (Figure 5, Figure 6 and Figure 7).
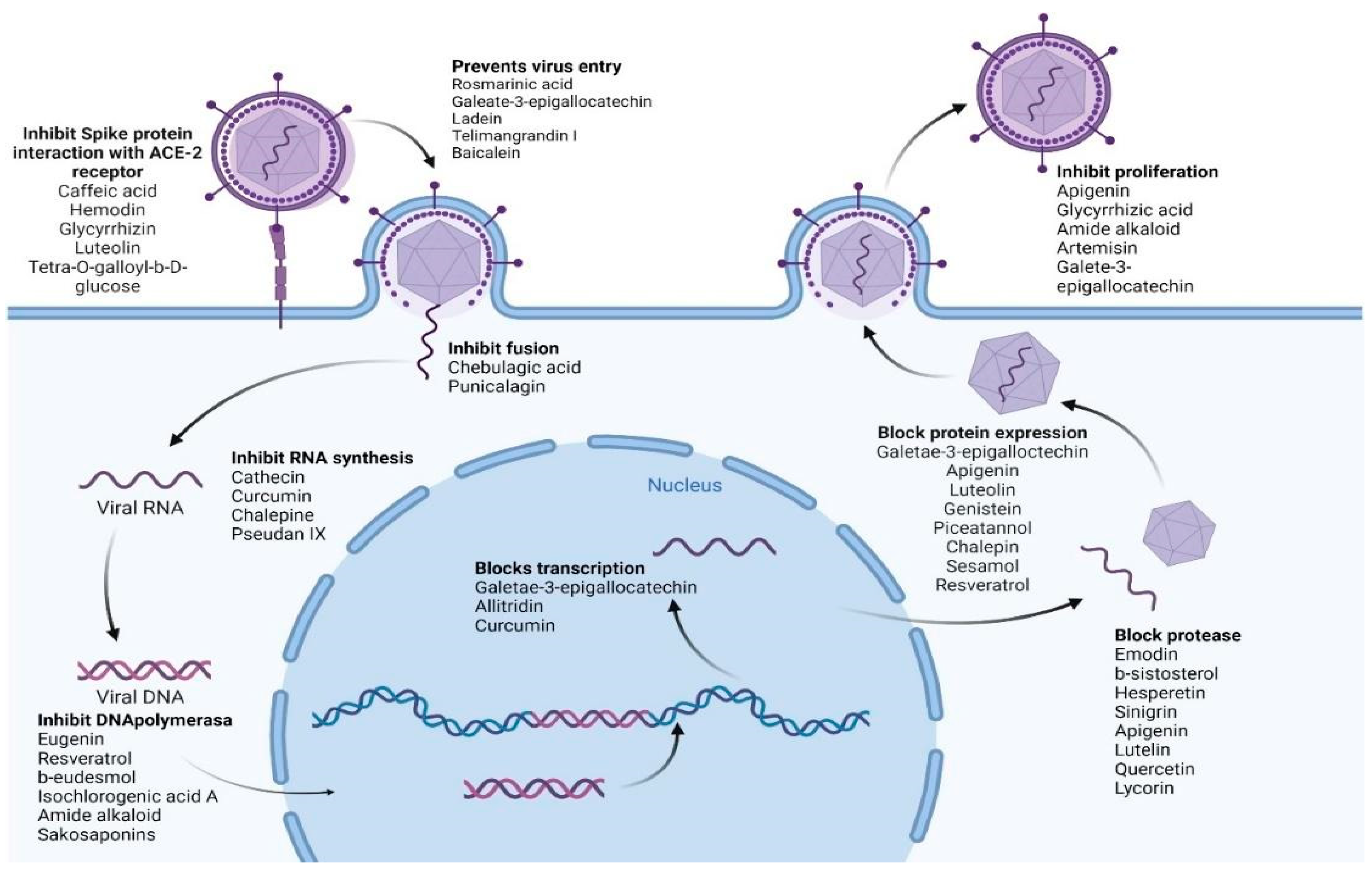
Figure 4. Various biocompounds’ potential antiviral mechanisms of action. Created with BioRender.com.
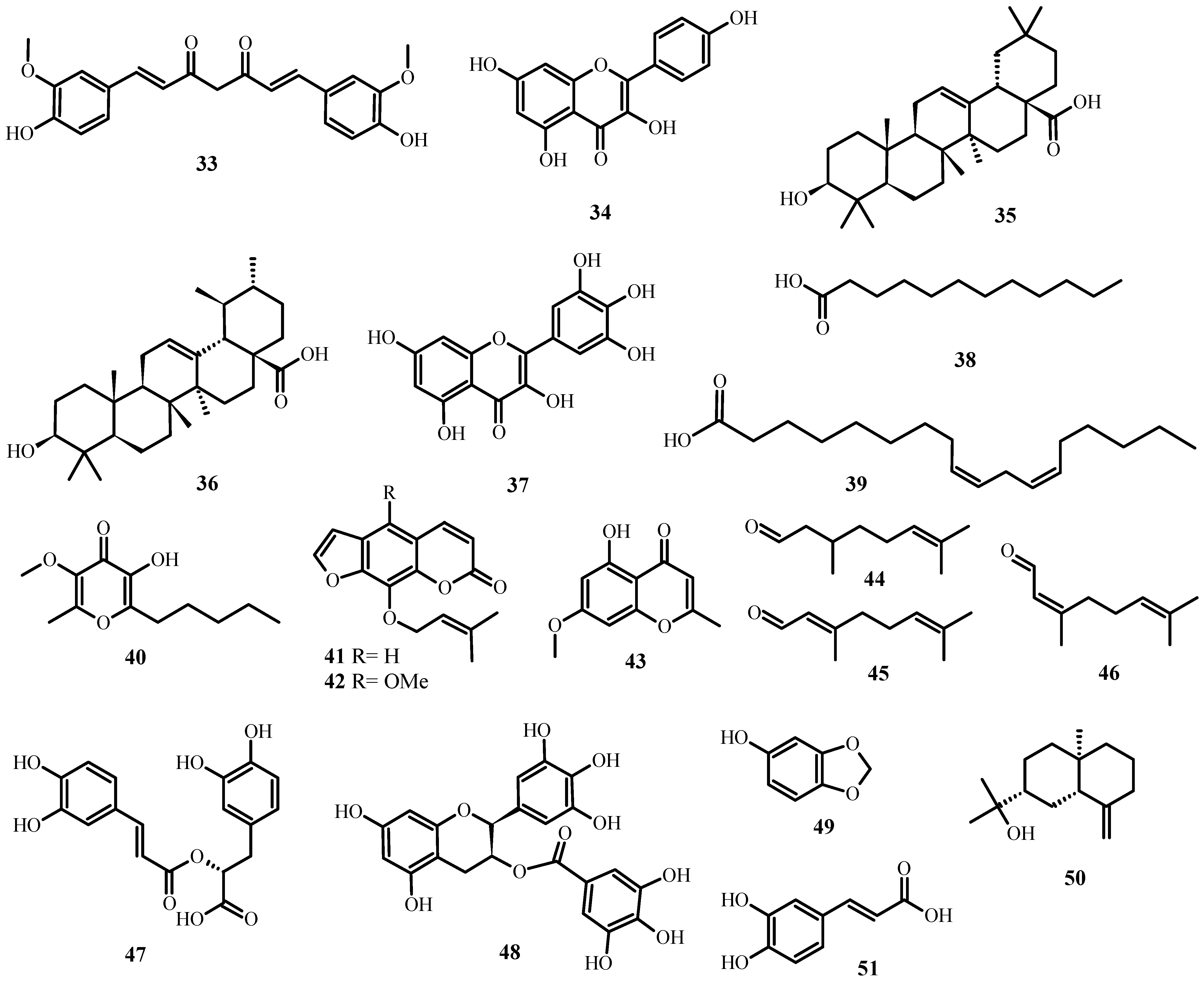
Figure 5. Antiviral biological compounds (Table 6).
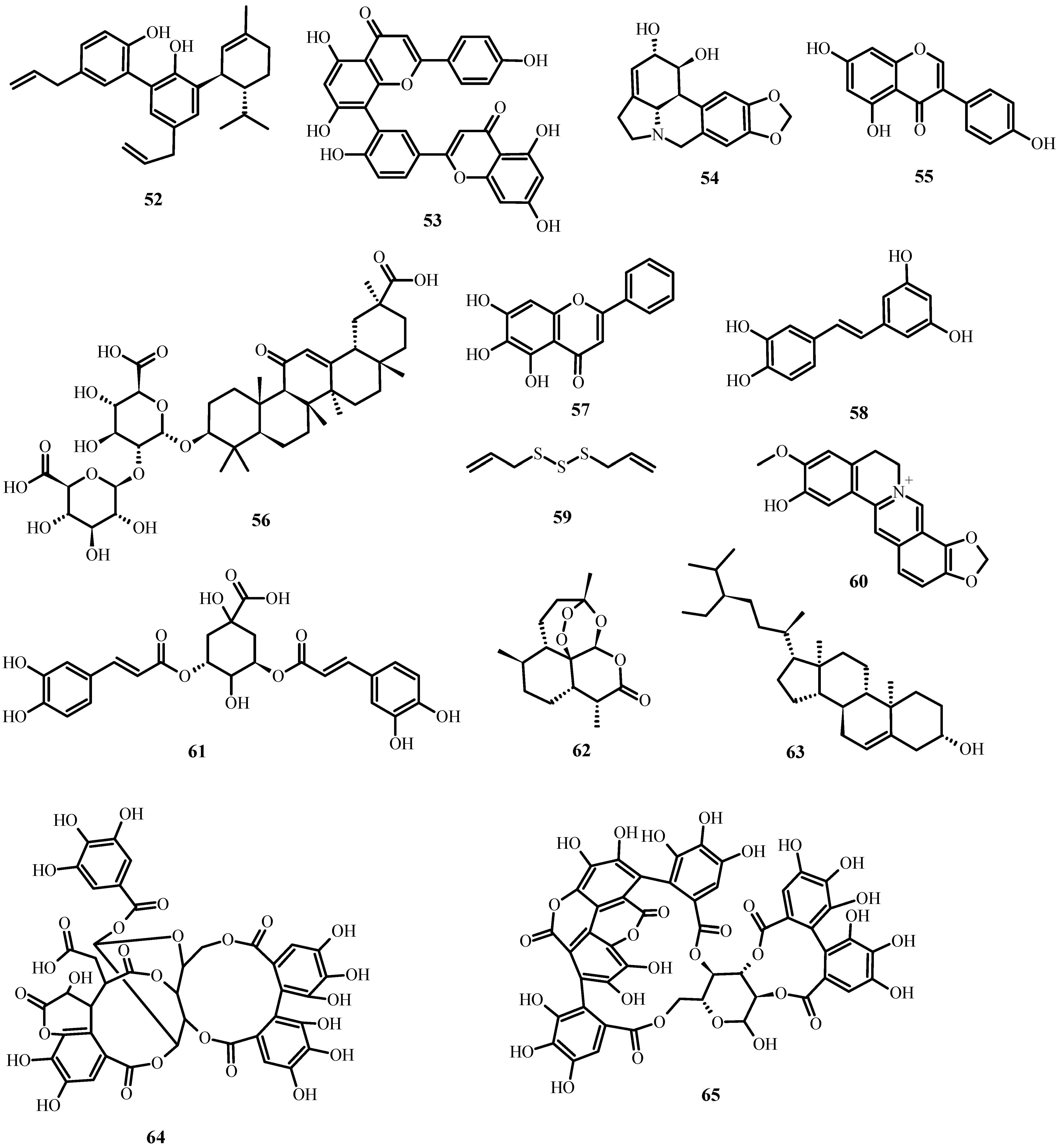
Figure 6. Antiviral biological compounds (Table 6).
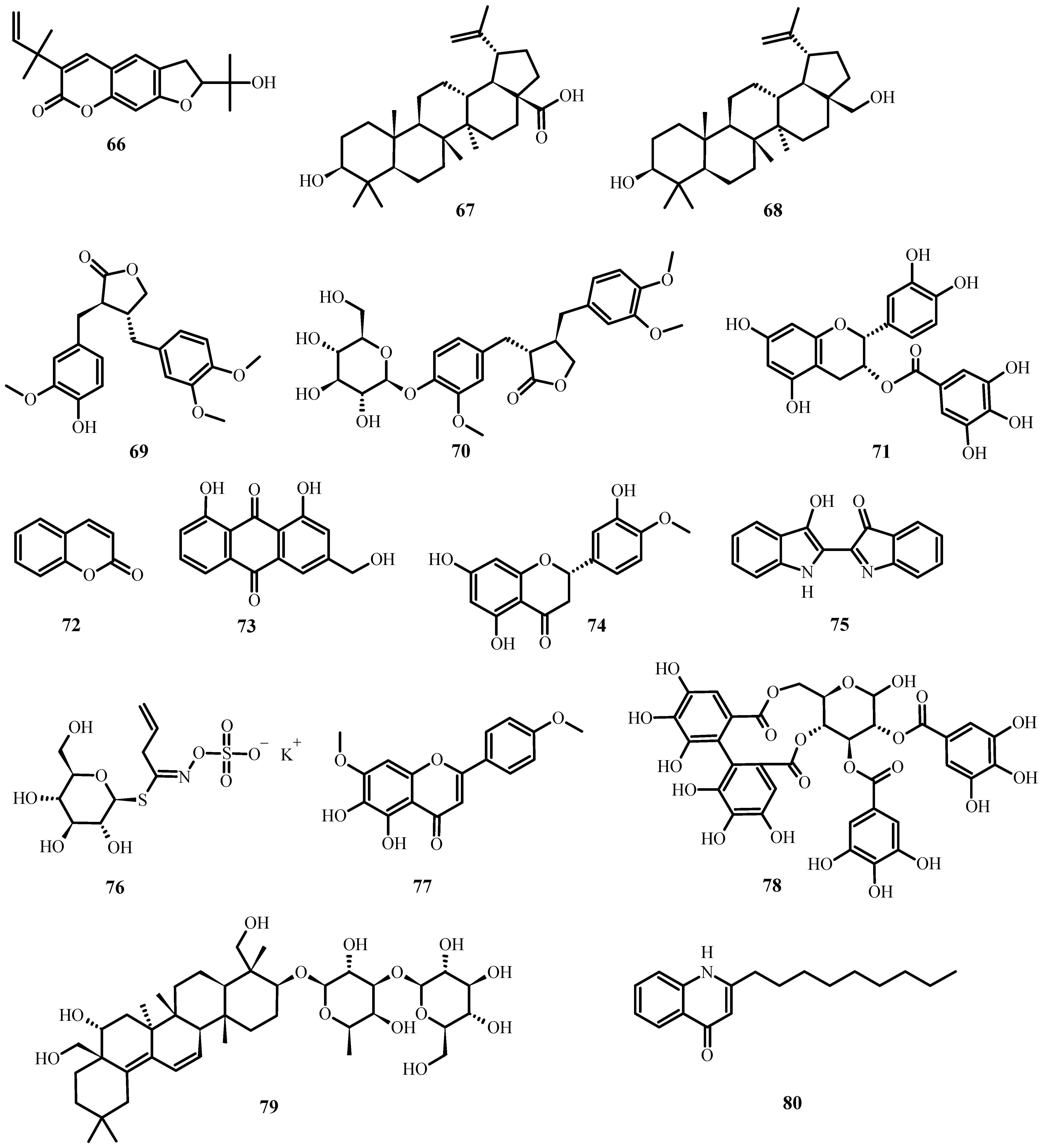
Figure 7. Antiviral biological compounds (Table 6).
2.6.2. Antiviral-Active Extracts for Respiratory Infections
The leading cause of morbidity in humans is viral respiratory tract infections, with rhinovirus, influenza, respiratory syncytial virus (RSV), and human coronavirus having the most significant impact.
Several extracts of medicinal plants exhibit antiviral activity in vitro; the ethanolic, ethyl acetate, and hexane extracts of Echinacea pallida var. angustifolia root inhibit rhinovirus replication [115]. The Echinacea purpurea ethanolic extract inhibits the invasion of HcoV-299E (coronavirus) into cells [116]. The ethanolic extract of Sambucus formosana Nakai seeds inhibits the binding of HCoV-NL63 (coronavirus) [117]. Aqueous extracts of Plantago asiatica and Clerodendrum trichotomun inhibit RSV (respiratory syncytial virus) replication [118].
2.6.3. Extracts and Biocompounds with Activity against Human Herpes Viruses
Several medicinal plant extracts have in vitro anti-herpes simplex activity: the hexane, dichloromethane, and methanolic extracts of Clinacanthus mutans and C. siamensis inhibit the formation of HS-1 and HS-2 viral plaques [119]. Caffeic acid and chlorogenic acid are inhibitors of HS replication [120]. Polygonum minus methanolic extract inhibits HS adhesion [121]. Aloe vera glycerol extract prevents HS-2 replication [122]. Lysimachia mauritiana ethanolic extract inhibits varicella-zoster virus replication [88].
Alkaloids, glycosides, taxol derivatives, terpenes, flavonoids, ellagitannin, catechin, phenolic acids, triterpenoids, monoterpenoids, and steroids have been identified as active against Herpes simplex types 1 and 2 [123].
Carvacrol (12), extracted from the essential oil of Mexican oregano (Lippia graveolens), demonstrates antiviral activity against RNA and DNA viruses (primarily herpes viruses) [124].
Coumarins imperatorin (41) and phellopterin (42), isolated from Angelica archeangelica L., exhibit antiviral activity against herpes simplex virus type 1 and, most likely, Coxsackievirus B3 [125].
Eugenin (43) is a biocompound extracted from Geum japonicum and Syzygium aromaticum. Eugenin (43) inhibits the DNA polymerase of the Herpes simplex virus, which appears to be its mechanism of action. Also, it inhibits Herpes simplex virus activity in both Vero cells and mice [126].
The monoterpene aldehydes citral a (45), citral b (46), and citronellal (44) are the biocompounds found to have anti-herpes virus activity in Melissa officinalis essential oil [127]. Moreover, rosmarinic acid (47) from the hydroalcoholic leaf extract of M. officinalis demonstrates anti-herpes simplex type 2 activity [128]. The potential mechanism of action is to prevent virus entry into cells [129].
2.6.4. Activity against Epstein-Barr Virus
Epstein–Barr (EBV) is a herpes virus that affects 90 percent of the world’s population and is linked to numerous immunological and neoplastic diseases.
Epigallocatechin-3-gallate (48), a catechin derived from Camellia sinensis, inhibits the spontaneous lytic infection of infected cells and blocks their transcription and protein expression via the ERK1/2 (extracellular-regulated kinase 12) and PI3-K/Akt (phosphatidylinositol-3-kinase) pathways [130].
The compounds sesamol (49) and resveratrol (2), along with sesame and sunflower essential oils, inhibit the early antigen activation in vitro of the Epstein–Barr virus [131].
Konoshima et al. [132] found that monoterpenylmagnolol (52) and β-eudesmol (50), extracted from Magnolia officinalis, inhibit replication (EBV) in Raji cells.
Berberine (15) is an alkaloid derived from several medicinal plants (Cortidis rhizome, Coptis chinensis, and Barnerini vulgaris) that inhibits cell proliferation and induces apoptosis in Epstein–Barr virus-infected cells via the inhibition of p-STAT3 and the overexpression of EBNA1 [133].
Curcumin (33) is highly effective at reducing TPA-, butyrate-, and TGF-b-induced levels of BZLF1 mRNA and TPA-induced luciferase mRNA, indicating that it inhibits three main EBV pathways [134].
Apigenin (29) inhibits the expression of the EBV lytic proteins Zta, Rta, EAD, and DNase in B and epithelial cells. In addition, it decreases the number of EBV-reactivating cells detectable via immunofluorescence analysis. Additionally, apigenin (29) has been found to significantly reduce EBV virus production [135].
Glycyrrhizic acid (56) (18-GL or GL) possesses a wide range of antiviral activities, pharmacological effects, and sites of action. In vitro, GL (56) inhibits Epstein–Barr virus (EBV) infection by interfering with an early stage in the EBV replication cycle (possibly attachment or penetration) [136].
The flavonoid luteolin (25) inhibits EBV reactivation significantly. In EBV-positive epithelial and B cell lines, 25 inhibits the expression of EBV-lytic gene-encoded proteins. In addition, it decreases the number of EBV-reactivating cells detected via immunofluorescence and virion production. Moreover, 25 decreases the activities of the promoters of the immediate–early genes Zta (Zp) and Rta (Rp). It inhibits the activity of Sp1-luc, indicating that the disruption of Sp1 binding is involved in the mechanism of inhibition [137].
2.6.5. Anti-Cytomegalovirus Activities
Human cytomegalovirus (hCMV) is a pervasive herpesvirus that causes a latent infection that persists throughout the host’s lifetime and can be reactivated when immunity is compromised.
Genistein (55) and baicalein (57) are antiviral flavonoids against HCMV. The primary mode of action of genistein’s antiviral activity against HCMV is to inhibit the function of immediate–early proteins. Baicalein’s antiviral activity against HCMV works primarily by inhibiting the kinase activity of EGFR to prevent viral entry [138].
Supplementation with piceatannol (58) inhibits the lytic changes caused by hCMV infection. In addition, piceatannol dose-dependently inhibits the expression of hCMV immediate–early (IE) and early (E) proteins and the replication of hCMV DNA [139].
Resveratrol (2) inhibits human cytomegalovirus DNA replication to undetectable levels during the second (late) phase of virus-induced phosphatidylinositol-3-kinase signaling and transcription factor activation [140].
Allitridin (59), a compound extracted from A. sativum, reduces the amount of viral DNA in cytomegalovirus-infected cells by inhibiting the transcription of the IE gene [141].
2.6.6. Anti-HIV Activity of Extracts and Biocompounds
Among the extracts that inhibit in vitro HIV activity or replication is an aqueous extract of Salvia miltiorrhiza that inhibits HIV-1 integrase [142]. Rhaphiolepsis indica methanolic extract inhibits its replication [143]. Acacia arabica’s n-butanol fraction inhibits the activity of viral proteases and Tat [144]. The Phyllanthus amarus ethanolic and aqueous extracts inhibit its replication [145]. The Olea europaea aqueous extract inhibits cell–cell infection [146]. Hyssopus officinalis L. aqueous extract inhibits its replication [147]. The reverse transcriptase is inhibited by the methanolic extract of Terminalia sericea [148], the n-hexane fraction of Phyllanthus emblica and Cassia occidentalis, and the pine cone extract of Pinus yunnanensis [149]; floral extracts of Calendula officinalis inhibit HIV-1 reverse transcriptase activity [150]. Cassine xylocarpa’s lupane-type pentacyclic triterpenoid also possesses anti-HIV activity [151].
2.6.7. Antiviral Activity of Extracts and Biocompounds against Hepatitis B and C Viruses
The secondary metabolites and extracts listed below have demonstrated in vitro activity against HBV (Hepatitis virus B): isochlorogenic acid A (61), obtained from Laggera alata, inhibits replication and decreases the stability of its core protein [152]. Amide alkaloids from Piper longum [153] and dehydrocheilanthifoline (60), isolated from Corydalis saxifolia, inhibit its replication [154]. Saikosaponins (Bupleurum species) inhibit the replication and expression of its surface antigen [155]; the ethanolic extract of Polygonum cuspidatum inhibits its surface antigen expression [156]; and curcumin (33) (Curcuma longa) decreases the expression of the PGC-1a coactivator, required for its transcription [157]. Glycyrrhizinic acid (56) (Glycyrrhiza glabra), artemisinin (62) (Artemisia annua), and LPRP-Et-97,543 compound (Liriope platyphylla) all inhibit its viral production [158]. On the other hand, epigallocatechin-3-gallate (48) (Camellia sinensis) inhibits its viral replication [159].
Concerning the hepatitis C virus, flavonolignans (Silybum marianum) possess antiviral and antioxidant properties [160], while curcumin (33) (Curcuma longa) inhibits its viral replication via the Akt-SREBP-1 pathway [161]. Epigallocatechin-3-gallate (48) [162] and ladanein (77) [163] inhibit viral entry; griffithsin inhibits viral cell–cell transmission [164], tellimagrandin I (78) (Rosa rugosa) inhibits viral invasion [158], chebulagic acid (64) and punicalagin (65) (Terminalia chebula) inhibit the viral particles necessary for their fusion and cell–cell transmission [165], saikosaponin B2 (79) (Bupleurum kaoi) prevents viral binding, and chalepine (66) and pseudan IX (80) (Ruta angustifolia) decrease viral protein synthesis and RNA replication [158][166].
Betulinic acid (67) and betulin (68), derived from Betula alba L., exhibit anti-hepatitis C virus activity. Shikov et al. (2011) [167] suggested that 68 can induce TNF-α expression and thereby enhance the Th1-type immune cell response in patients with chronic hepatitis C virus.
2.6.8. Anti-Influenza Activity of Extracts and Biomolecules
Moradi (2019) [168] reported that ethanolic and polyphenolic extracts of Punica granatum inhibit influenza replication and virions. Geranium sanguineum polyphenolic, methanolic, and ethanolic extracts have antiviral properties [169]. Glycyrrhizin (56) from Glycyrrhiza glabra induces the apoptosis of H5N1-infected cells [170]; polyphenols from Chenomeles sinensis inhibit the binding of its hemagglutinins [171]; and the Sambucus nigra fruit inhibits viral entry and modulates cytokine release. It has been shown in other studies to inhibit hemagglutins and the replication of influenza viruses: A/Shangdong 9/93 (H3N2), A/Beijing 32/92 (H3N2), A/Texas 36/91 (HlNl), A/Singapore 6/86 (HlNl), type B/Panama 45/90, B/Yamagata 16/88, and B/Ann Arbor [172].
The Phyllanthus embolica aqueous extract inhibits hemagglutinin and viruses in infected cells [173]. Catechin derived from Camellia sinensis inhibits both RNA synthesis and neuraminidase activity [174].
Arctigenin (69) and arcitiin (70), extracted from the fruits of Arctium lappa L., exhibit potent anti-influenza A virus activity in vitro [175].
Echinacea extract is active against influenza A/B viruses (H3N2, H1N1, H5N1, H7N7, and S-OIV), Respiratory Syncytial Virus, and Herpes Simplex [176]. On the other hand, it also induces the production of IL-6 and IL-8 (CXCL8) and other cytokines with antiviral properties [177]. In a clinical trial, it was demonstrated to be as effective as oseltamivir in reducing influenza symptoms if administered at the onset of the disease [178].
On the other hand, the monoterpene aldehydes citral a (45) and citral b (46), from Melissa officinalis, exhibit synergistic activity with oseltamivir against the H9N2 influenza virus [179].
Wyde et al. [180] found that polyphenolic polymers derived from the Euphorbiaceae shrub are active in vitro against parainfluenza virus type 3, Respiratory Syncytial Virus, and influenza viruses.
2.6.9. Extracts In Vitro Possess Anti-Papillomavirus Activity
Their growth is inhibited by polyphenon E (71) (poly E) and epigallocatechin gallate (48) from Camellia sinensis [181]. Artemisinin (62) (Artemisia absintium) inhibits the expression of HPV-39, induces apoptosis, and reduces the proliferation of infected cells in ME-180 cells [182]. Curcumin (33) (Curcuma longa L.) has been utilized to boost immunity against HPV. Hamamelis virginiana tannins inhibit HPV-16, Ficus religiosa aqueous extract induces the apoptosis of HPV-16 and 18 infected cervical cells, and the Phyllanthus emblica fruit inhibits HPV-16 and 18 carcinogenic gene expression. The chloroplast leaf extract of Bryophyllum pinnatum inhibits HPV-18 transcription in cervical cancer cells, whereas the soluble extract of Pinellia pedatisecta inhibits HPV-E6 expression in multiple cell lines [183].
2.6.10. In Vitro Activity of Extracts against Dengue and Chikungunya Viruses
Coumarin (72) and the ether extract of Alternanthera philoxeroides [184], as well as aqueous and chloroform extracts of Carioca papaya [185], inhibit dengue virus. Sambucus nigra methanolic extract protects against dengue serotype 2 [186].
Vernonia amygdalina ethyl acetate extract reduces the Chikungunya viral burden [187]. Chikungunya helicases and proteases are inhibited by aqueous extracts of Picrorhiza kurrooa, Ocimum tenuiflorum, and Terminalia chebula [188].
2.6.11. Antiviral Activity of In Vitro Extracted Compounds against SARS-CoV
The following organisms were evaluated for their anti-SARS-CoV-1 activity: Lycoris radiate, Artemisia annua, Pyrrosia lingua, Lindera aggregata, Isatis indigotica (inhibition of 3CL protease) [189], Rheum officinale Bail, Polygonum multiforum Thunb. (inhibit ACE2 protein interaction with spike protein) [190], Gentiana scabana, Dioscorea batatas, Casssia tora, Taxillus chinensis, Cibotium barometz (inhibit 3CL protease) [191], and ethanolic extracts of Anthemis hyalina, Nigella sativa, and Citrus sinensis (increase IL-8, modify TRPA, TRPM, and TRPV gene expression) [192]. Some purified secondary metabolites that show activity against SARS-CoV are as follows: aloe-emodin (73), β-sitosterol (63), hesperetin (74), indigo (75), and sinigrin (76) (obtained from Isatis indigotica) [193], amentoflavone (53), apigenin (29), luteolin (25), quercetin (10) (obtained from Torreya nucifera), which inhibit 3CL protease [194], and lycorine (54) (obtained from Lycoris radiata) [189].
In the case of SARS-CoV-2 (which causes COVID-19), the following secondary metabolites (Figure 8) may be advantageous [195]:
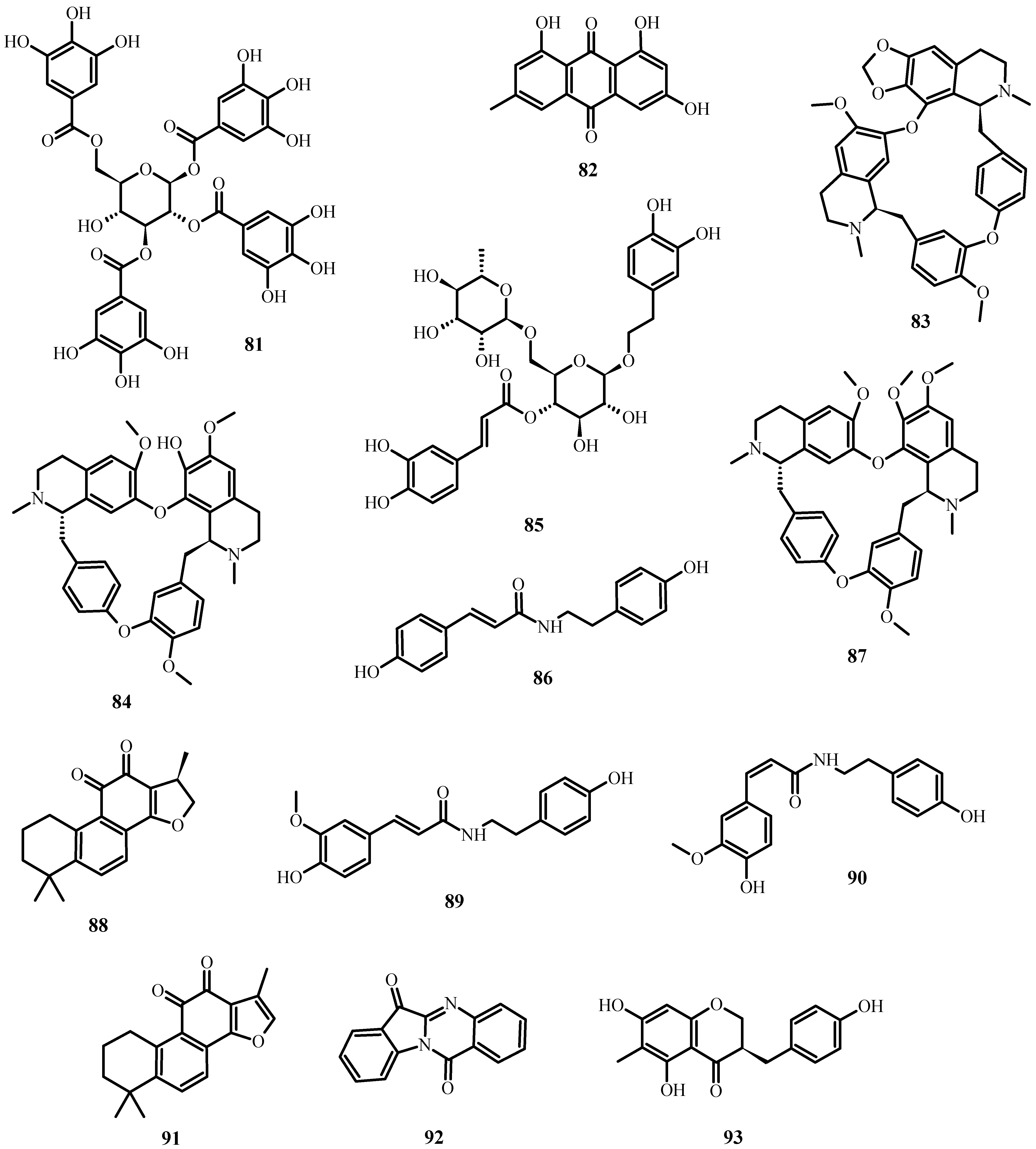
Figure 8. Natural products reported to have anti-SARS-CoV-2 activity.
-
To inhibit the binding of the spike protein to the ACE-2 receptor: caffeic acid (51), emodin (82), glycyrrhizin (56), luteolin (25), and tetra-O-galloyl-β-D-glucose (81).
-
To prevent virus transcription: cepharanthin (83), fangquinoline (84), forystoside A (85), tetrandin (87), coumaroyltyramine (86), cryptoansionone (88), kaempferol (34), moupinamide (89), N-cis-feruloyltyramine (90), quercetin (10), tanshinone IIa (91), and tryptanthrine (92).
-
To inhibit viral translation: tryptanthrine (92).
-
To inhibit the cellular discharge of virions: emodin (82) and kaempferol (34).
Table 5. Antiviral extracts derived from plants.
| Plant | Extract | Virus | Possible Antiviral Mechanism | References |
|---|---|---|---|---|
| Echinacea pallida var. angustifolia | Hexane | Rhinovirus | Impedes replication. | [115] |
| Echinacea purpurea | Ethanolic | Coronavirus HcoV-299E | Prevents the invasion of cells. | [116] |
| Sambucus formosana Nakai | Ethanolic | HCoV-NL63 (coronavirus) | Prevents bonding. | [117] |
| Plantago asiatica | Aqueous extract | Respiratory syncytial virus | Replication inhibition. | [118] |
| Clerodendrum trichotomun | Aqueous extract | Respiratory syncytial virus | Replication inhibition. | [118] |
| Clinacanthus mutans Clinacanthus siamensis |
Hexane, dichloromethane, and methanolic | Herpes simplex-1 and 2 | Inhibit viral plaques. | [119] |
| Polygonum minus | Methanolic | Herpes simplex-1 and 2 | Inhibits adhesion. | [121] |
| Aloe vera | Glycerol | Herpes simplex 2 | Impedes replication. | [122] |
| Lysimachia mauritania | Ethanolic extract | Varicella-zoster | Impedes replication. | [88] |
| Sesamum indicum Helianthus annuus |
Sesame essential oil and Sunflower essential oil | Epstein-Barr Virus | Inhibit precocious antigen activation. | [131] |
| Salvia miltiorrhiza | Aqueous extract | HIV-1 | Interferes with integrase activation. | [142] |
| Rhaphiolepsis indica | Methanolic extract | HIV-1 | Impedes replication. | [143] |
| Acacia arabica | N-butanol fraction | HIV-1 | Inhibits viral proteases and Tat activity. | [144] |
| Phyllanthus amarus Schum. | Ethanolic and aqueous extract | HIV-1 | Impedes replication. | [145] |
| Olea europaea | Aqueous extract | HIV-1 | Prevents infections between cells. | [146] |
| Hyssopus officinalis L. | Aqueous extract | HIV-1 | Inhibits replication. | [147] |
| Polygonum cuspidatum | Ethanolic extract | Hepatitis virus B | Inhibits surface antigen expression. | [156] |
| Punica granatum | Ethanolic and polyphenolic extracts | Influenza virus | Inhibits influenza replication and virions. | [168] |
| Geranium sanguineum | Polyphenolic, methanolic, and ethanolic | Influenza virus | No study. | [169] |
| Chenomeles sinensis | Polyphenols | Influenza virus | Inhibits the attachment of its hemagglutinins. | [171] |
| Sambucus nigra | Aqueous extract | Influenza virus | Modulates cytokine release and inhibits viral entrance. | [172] |
| Phyllanthus emblica | Aqueous extract | Influenza virus | Prevents hemagglutinins and viruses from infecting infected cells. | [173] |
| Echinacea purpurea | Aqueous extract | Influenza A/B viruses H3N2, H1N1, H5N1, H7N7, and S-OIV | Induces IL-6 and IL-8 production. | [176] |
| Euphorbiacea shrub | Polyphenolic polymers | Influenza | No study. | [180] |
| Ficus religiosa | Aqueous extract | Papillomavirus | HPV-16 apoptosis is induced. | [183] |
| Bryophyllum pinnatum | Chloroplast extract | Papillomavirus | Suppresses HPV-18 transcription. | [183] |
| Pinellia pedatisecta | Soluble extract | Papillomavirus | Inhibits the HPV-E6 expression in multiple cell lines. | [183] |
| Carioca papaya | Aqueous and chloroplast extract | Chikungunya | Stops the dengue virus. | [185] |
| Sambucus nigra | Methanolic extract | Dengue serotype 2 | Defends against infection. | [186] |
| Vernonia amygdalina | Ethyl acetate extract | Chikungunya | Minimizes the viral burden. | [187] |
| Picrorhiza kurrooa Ocimum tenuiflorum Terminalia chebula |
Aqueous extracts | Chikungunya | Block helicases and proteases. | [188] |
| Lycoris radiate, Artemisia annua, Pyrrosia lingua, Lindera aggregata, and Isatis indigotica | Different extracts | SARS-CoV-1 | Obstruct 3CL protease. | [189] |
| Rheum officinale Bail, Polygonum multiforum Thunb | Different extracts | SARS-CoV-1 | Inhibit the interaction between ACE2 and spike proteins. | [190] |
| Gentiana scabana, Dioscorea batatas, Casssia tora, Taxillus chinensis, and Cibotium barometz | Different extracts | SARS-CoV-1 | Prevent 3CL protease. | [191] |
| Anthemis hyalina, Nigella sativa, and Citrus sinensis | Ethanolic extracts | SARS-CoV-1 | Increase IL-8 and modulate gene expression of TRPA, TRPM, and TRPV. | [192] |
Table 6. Antiviral biological compounds.
| Secondary Metabolite Class | Biocompound (Species) | Virus | Potential Antiviral Mechanism | Reference |
|---|---|---|---|---|
| Menthane monoterpenoids | Carvacrol (12) (Lippia graveolens) | Herpes viruses | No study. | [124] |
| Furocoumarin | Imperatorin (41) and phellopterin (42) (Angelica archangelica) | Herpes simplex virus type 1 Coxsackievirus B3 |
No study. | [125] |
| Chromone | Eugenin (43) (Geum japonicum, Syzygium aromaticum) |
Herpes simplex virus | Prevents DNA polymerase. | [126] |
| Cinnamic acid derivative | Rosmarinic acid (47) (M. officinalis) |
Herpes simplex type 2 | Prevents virus entry into cells. | [128] |
| Flavan-3-ol | Epigallocatechin-3-gallate (48) (Camellia sinensis) | Epstein–Barr Virus | Blocks transcription and protein expression via ERK1/2 (extracellular-regulated-kinase 12) and PI3-K/Akt (phosphatidylinositol-3-kinase) pathways. | [130] |
| Phenol, Monomeric stilbene | Sesamol (49), resveratrol (2) (Sesamum indicum) | Epstein–Barr Virus | Inhibit early antigen activation. | [131] |
| Isoquinoline alkaloid | Berberine (15) (Barnerini vulgaris) | Epstein–Barr Virus | Inhibits cell proliferation and induces apoptosis in Epstein–Barr virus-infected cells by inhibiting p-STAT3. | [133] |
| Linear diarylheptanoid | Curcumin (33) (Curcuma longa) | Epstein–Barr Virus | Inhibits TPA-, butyrate-, and TGF-b induced levels of BZLF1 mRNA | [134] |
| Flavone | Apigenin (29) (purchased from Sigma-Aldrich Co., St. Louis, MO, USA) | Epstein–Barr Virus | Inhibits lytic proteins Zta, Rta, EAD, and DNase in B and epithelial cells and reduces the production of EBV viruses. | [135] |
| Oleanane triterpenoid | Glycyrrhizic acid (56) (Glycyrrhiza radix) | Epstein–Barr Virus | Interferes with the initial phase of EBV replication. | [136] |
| Flavone | Luteolin (25) (purchased from Sigma-Aldrich Co.) | Epstein–Barr Virus | Inhibits the expression of proteins encoded by the EBV lytic gene. | [137] |
| Isoflavone | Genistein (55) (purchased from Sigma-Aldrich) | Cytomegalovirus | Inhibits immediate-early (ie) protein function. | [138] |
| Flavone | Baicalein (57) (purchased from Sigma-Aldrich) | Cytomegalovirus | Inhibits EGFR’s kinase activity to prevent viral entry. | [138] |
| Monomeric stilbene | Piceatannol (58) (purchased from Sigma-Aldrich) | Cytomegalovirus | Inhibits the lytic modifications and expression of hCMV early (E) and immediate–-early (IE) proteins. | [139] |
| Monomeric stilbene | Resveratrol (2) (purchased from Sigma-Aldrich) | Cytomegalovirus | Reduces DNA replication. | [140] |
| Sulfide | Allitridin (59) (A. sativum) | Cytomegalovirus | Inhibits the IE genes’ transcription. | [141] |
| Neolignan | Monoterpenylmagnolol (52) and β-eudesmol (50) (Magnolia officinalis) | Epstein–Barr Virus | Impede replication. | [132] |
| Cinnamic acid derivative | Isochlorogenic acid A (61) (Laggera alata) | Hepatitis virus B | Impedes replication. | [152] |
| Alkaloid | Amide alkaloids (Piper longum) | Hepatitis virus B | Inhibit replication and surface antigen expression. | [153] |
| Saponin | Saikosaponins (Bupleurum species) | Hepatitis virus B | Inhibit replication and surface antigen expression. | [155] |
| Protoberberine alkaloid | Dehydrocheilanthifoline (60) (Corydalis saxifolia) | Hepatitis virus B | Prevents reproduction. | [154] |
| Linear diarylheptanoid | Curcumin (33) (Curcuma longa) | Hepatitis virus B | Decreases Transcription. |
[157] |
| Oleanane triterpenoid | Glycyrrhizinic acid (56) (Glycyrrhiza glabra) | Hepatitis virus B | Prevents viral reproduction. | [158][196] |
| Sesquiterpene lactone | Artemisinin (62) (Artemisia annua) | Hepatitis virus B | Prevents viral reproduction. | [158][196] |
| Isoflavonoid | LPRP-Et-97543 (93) (Liriope platyphylla) | Hepatitis virus B | Prevents viral reproduction. | [158][196] |
| Flavan-3-ol | Epigallocatechin-3-gallate (48) (Camellia sinensis) | Hepatitis virus B | Prevents viral reproduction. | [159] |
| Lignan | Flavonolignans (Silybum marianum) | Hepatitis C virus | No study. | [160] |
| Linear diarylheptanoid | Curcumin (33) (Curcuma longa) | Hepatitis C virus | Inhibits viral replication by blocking Akt-SREBP-1. | [161] |
| Flavan-3-ol | Epigallocatechin-3-gallate (48) (Camellia sinensis) | Hepatitis C virus | Inhibits viral introduction. | [162] |
| Flavone | Ladanein (77) (Marrubium peregrinum) | Hepatitis C virus | Inhibits viral introduction. | [163] |
| Peptide | Recombinant Griffithsin (Nicotiana benthamiana) | Hepatitis C virus | Inhibits viral cell–cell transmission. | [164] |
| Gallotannin | Tellimagrandin I (78) (Rosae rugosae) | Hepatitis C virus | Prevents viral penetration. | [158] |
| Benzopyran tannin and phenol | Chebulagic acid (64) and punicalagin (65) (Terminalia chebula Retz) | Hepatitis C virus | Inhibit fusion and cell–cell transmission. | [165] |
| Oleanane triterpenoid | Saikosaponin B2 (79) (Bupleurum kaoi) | Hepatitis C virus | Prevents viral attachment. | [158] |
| Furocoumarin, Quinoline alkaloid | Chalepine (66), pseudan IX (80) (Ruta angustifolia) | Hepatitis C virus | Reduce viral protein synthesis and viral RNA replication. | [158] |
| Lupane triterpenoids | Betulinic acid (67) and betulin (68) (Betula alba L) | Hepatitis C virus | Induce expression of TNF-α. | [167] |
| Oleanane triterpenoid | Glycyrrhizin (56) (Glycyrrhiza glabra) | Influenza virus | Initiates cell death in H5N1-infected cells. | [170] |
| Catechin | Catechins (Camellia sinensis) | Influenza virus | Inhibit both RNA synthesis and neuraminidase activity. | [174] |
| Dibenzylbutyrolactone lignans | Arctigenin (69) and arcitiin (70) (Arctium lappa) | Influenza virus | Anti-influenza A virus in vitro activity. | [175] |
| Monoterpenaldehydes | Citral a (45) and citral b (46) (Melissa officinalis) | H9N2 influenza virus | Have synergistic activity with oseltamivir. | [179] |
| Flavan-3-ols | Polyphenon E (poly E) (71) and epigallocatechin gallate (48) (Camellia sinensis) | Papillomavirus | Impede growth. | [181] |
| Sesquiterpene lactone | Artemisinin (62) (Artemisia absintium) | Papillomavirus | In ME-180 cells, this compound inhibits the expression of HPV-39, induces apoptosis, and reduces the proliferation of infected cells. | [182] |
| Tannin | Tannins (Hamamelis virginiana) | Papillomavirus | Inhibit HPV-16 | [183] |
| Benzopyrone | Coumarin (33) (Alternanthera philoxeroides) | Chikungunya | Stops the dengue virus. | [184] |
| Anthraquinone, Stigmastane steroid, Flavanone, Anthranilic acid alkaloid, Glucosinolate | Emodin (82), β-sistosterol (63), hesperetin (74), indigo (75), and sinigrin (76) (Isatis indigotica) | SARS-CoV-1 | Block the 3CL protease. | [193] |
| Flavones, Flavonol | Amentoflavone (53), apigenin (29), luteolin (25), quercetin (10) (Torreya nucifera) | SARS-CoV-1 | Block the 3CL protease. | [194] |
| Indolizidine alkaloid | Lycorine (54) (Lycoris radiata) | SARS-CoV-1 | Block 3CL protease. | [189] |
| Cinammic acid derivative, Anthraquinone, Oleanane triterpenoid, Flavonoid, Gallotannin |
Caffeic acid (51), emodin (82), glycyrrhizin (56), luteolin (25), and tetra-O-galloyl-β-D-glucose (81) | SARS-CoV-2 | Inhibit the spike protein’s interaction with the ACE-2 receptor. | [195] |
2.6.12. Molecules with Antiviral Activity Identified In Silico
Computational models enable to simulate the interaction between the biocompound and the virus’s target molecule [197]. Quercetin-7-O-glucoside inhibits influenza virus RNA polymerase. Quercetagetin, a flavonoid with activity against HVC through the inhibition of RNA bound to NS5B non-structural polymerase [198], naringenin, and quercetin, could inhibit hepatitis C virus proteases [199], and β-amyrin could inhibit hepatitis D virus proteases [200].
Luteolin could block SARS-CoV-2 entrance into cells [201]; isothymol and curcumin can block angiotensin-converting enzyme receptor (ACE2) activity [202]; gingerol binds to the spike protein; and quercetin with proteases [203], enterodiol, taxifolin, eriodictyol, leucopelargonidin, morin, and myricetin were found to exhibit remarkable binding affinities against the major protease (Mpro) and potato-like protease (PLpro) [204].
This entry is adapted from the peer-reviewed paper 10.3390/plants12233997
References
- Zhong, C.; Zhang, F.; Yao, J.; Zhu, Y.; Zhu, N.; Zhang, J.; Ouyang, X.; Zhang, T.; Li, B.; Xie, J.; et al. New Antimicrobial Peptides with Repeating Unit against Multidrug-Resistant Bacteria. ACS Infect. Dis. 2021, 7, 1619–1637.
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent Bacterial Infections and Persister Cells. Nat. Rev. Microbiol. 2017, 15, 453–464.
- Luo, G.G.; Gao, S.J. Global Health Concerns Stirred by Emerging Viral Infections. J. Med. Virol. 2020, 92, 399–400.
- Ji, C.; Liu, N.; Tu, J.; Li, Z.; Han, G.; Li, J.; Sheng, C. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect. Dis. 2020, 6, 768–786.
- O’Neill, J. Tackling Drug-Resistance Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Zhu, Y.; Huang, W.E.; Yang, Q. Clinical Perspective of Antimicrobial Resistance in Bacteria. Infect. Drug Resist. 2022, 15, 735–746.
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910.
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R.; et al. Antimicrobial Resistance Research in a Post-Pandemic World: Insights on Antimicrobial Resistance Research in the COVID-19 Pandemic. J. Glob. Antimicrob. Resist. 2021, 25, 5–7.
- Laxminarayan, R. The Overlooked Pandemic of Antimicrobial Resistance. Lancet 2022, 399, 606–607.
- Rizvi, S.G.; Ahammad, S.Z. COVID-19 and Antimicrobial Resistance: A Cross-Study. Sci. Total Environ. 2022, 807, 150873.
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40.
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501.
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200.
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258.
- Srivastava, J.; Chandra, H.; Nautiyal, A.R.; Kalra, S.J.S. Antimicrobial Resistance (AMR) and Plant-Derived Antimicrobials (PDAms) as an Alternative Drug Line to Control Infections. 3 Biotech 2014, 4, 451–460.
- Esmael, A.; Hassan, M.G.; Amer, M.M.; Abdelrahman, S.; Hamed, A.M.; Abd-raboh, H.A.; Foda, M.F. Antimicrobial Activity of Certain Natural-Based Plant Oils against the Antibiotic-Resistant Acne Bacteria. Saudi J. Biol. Sci. 2020, 27, 448–455.
- Intorasoot, A.; Chornchoem, P.; Sookkhee, S.; Intorasoot, S. Bactericidal Activity of Herbal Volatile Oil Extracts against Multidrug Resistant Acinetobacter Baumannii. J. Intercult. Ethnopharmacol. 2017, 6, 1.
- Dharmaratne, M.P.J.; Manoraj, A.; Thevanesam, V.; Ekanayake, A.; Kumar, N.S.; Liyanapathirana, V.; Abeyratne, E.; Bandara, B.M.R. Terminalia Bellirica Fruit Extracts: In-Vitro Antibacterial Activity against Selected Multidrug-Resistant Bacteria, Radical Scavenging Activity and Cytotoxicity Study on BHK-21 Cells. BMC Complement. Altern. Med. 2018, 18, 325.
- Khan, S.; Imran, M.; Imran, M.; Pindari, N. Antimicrobial Activity of Various Ethanolic Plant Extracts against Pathogenic Multi-Drug Resistant Candida spp. Bioinformation 2017, 13, 67–72.
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.-R.; Quave, C.L. Antibacterial Properties of Medicinal Plants From Pakistan against Multidrug-Resistant ESKAPE Pathogens. Front. Pharmacol. 2018, 9, 815.
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231.
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of Bactericidal Action of Eugenol against Escherichia coli. J. Herb. Med. 2021, 26, 100406.
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723.
- Liang, H.; Huang, Q.; Zou, L.; Wei, P.; Lu, J.; Zhang, Y. Methyl Gallate: Review of Pharmacological Activity. Pharmacol. Res. 2023, 194, 106849.
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218.
- Ferdes, M. Antimicrobial Compounds from Plants. In Fighting Antimicrobial Resistance; Budimir, A., Ed.; IAPC Publishing: Zagreb, Croatia, 2018; pp. 243–271. ISBN 978-953-56942-6-7.
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494.
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phytother. Res. 2019, 33, 13–40.
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and Anti-Biofilm Properties of Carvacrol Alone and in Combination with Cefixime against Escherichia coli. BMC Microbiol. 2023, 23, 55.
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Wang, X.; Sun, R.; Guo, W.; Yang, S.; Li, H.; Gong, G. Antibacterial Mechanism of Thymol against Enterobacter sakazakii. Food Control 2021, 123, 107716.
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of Biofilm Formation of Pseudomonas aeruginosa by Caffeine: A Potential Approach for Sustainable Management of Biofilm. Arch. Microbiol. 2020, 202, 623–635.
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; Li, L.; et al. Antibacterial Activity and Mechanism of Berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015, 8, 5217–5223.
- Mohanad, J.K.; Azhar, A.S.; Imad, H.H. Evaluation of Anti-Bacterial Activity and Bioactive Chemical Analysis of Ocimum basilicum Using Fourier Transform Infrared (FT-IR) and Gas Chromatography-Mass Spectrometry (GC-MS) Techniques. J. Pharmacogn. Phytother. 2016, 8, 127–146.
- Choo, J.H.; Rukayadi, Y.; Hwang, J.-K. Inhibition of Bacterial Quorum Sensing by Vanilla Extract. Lett. Appl. Microbiol. 2006, 42, 637–641.
- Ohene-Agyei, T.; Mowla, R.; Rahman, T.; Venter, H. Phytochemicals Increase the Antibacterial Activity of Antibiotics by Acting on a Drug Efflux Pump. MicrobiologyOpen 2014, 3, 885–896.
- Castro, F.A.V.; Mariani, D.; Panek, A.D.; Eleutherio, E.C.A.; Pereira, M.D. Cytotoxicity Mechanism of Two Naphthoquinones (Menadione and Plumbagin) in Saccharomyces cerevisiae. PLoS ONE 2008, 3, e3999.
- Ghosh, J.; Sil, P.C. Arjunolic Acid: A New Multifunctional Therapeutic Promise of Alternative Medicine. Biochimie 2013, 95, 1098–1109.
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the Antibiofilm and Antiquorum Sensing Activities of Eucalyptus globulus Essential Oil and Its Main Component 1,8-Cineole against Methicillin-Resistant Staphylococcus aureus Strains. Microb. Pathog. 2018, 118, 74–80.
- McLean, R.J.C.; Pierson, L.S.; Fuqua, C. A Simple Screening Protocol for the Identification of Quorum Signal Antagonists. J. Microbiol. Methods 2004, 58, 351–360.
- Carranza, M.G.; Sevigny, M.B.; Banerjee, D.; Fox-Cubley, L. Antibacterial Activity of Native California Medicinal Plant Extracts Isolated from Rhamnus Californica and Umbellularia Californica. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 29.
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal Plant Products Targeting Quorum Sensing for Combating Bacterial Infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743.
- Salem, M.Z.; Ali, H.M.; El-Shanhorey, N.A.; Abdel-Megeed, A. Evaluation of Extracts and Essential Oil from Callistemon viminalis Leaves: Antibacterial and Antioxidant Activities, Total Phenolic and Flavonoid Contents. Asian Pac. J. Trop. Med. 2013, 6, 785–791.
- Paredes, A.; Leyton, Y.; Riquelme, C.; Morales, G. A Plant from the Altiplano of Northern Chile Senecio nutans, Inhibits the Vibrio cholerae Pathogen. SpringerPlus 2016, 5, 1788.
- Alvin, A.; Miller, K.I.; Neilan, B.A. Exploring the Potential of Endophytes from Medicinal Plants as Sources of Antimycobacterial Compounds. Microbiol. Res. 2014, 169, 483–495.
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68.
- Botelho, M.A.; Nogueira, N.A.P.; Bastos, G.M.; Fonseca, S.G.C.; Lemos, T.L.G.; Matos, F.J.A.; Montenegro, D.; Heukelbach, J.; Rao, V.S.; Brito, G.A.C. Antimicrobial Activity of the Essential Oil from Lippia sidoides, Carvacrol and Thymol against Oral Pathogens. Braz. J. Med. Biol. Res. 2007, 40, 349–356.
- Subramani, R.; Narayanasamy, M.; Feussner, K.-D. Plant-Derived Antimicrobials to Fight against Multi-Drug-Resistant Human Pathogens. 3 Biotech 2017, 7, 172.
- Swamy, M.; Sinniah, U. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547.
- Proestos, C.; Chorianopoulos, N.; Nychas, G.-J.E.; Komaitis, M. RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity. J. Agric. Food Chem. 2005, 53, 1190–1195.
- Rojas, A.; Hernandez, L.; Pereda-Miranda, R.; Mata, R. Screening for Antimicrobial Activity of Crude Drug Extracts and Pure Natural Products from Mexican Medicinal Plants. J. Ethnopharmacol. 1992, 35, 275–283.
- Song, X.; Xia, Y.-X.; He, Z.-D.; Zhang, H.-J. A Review of Natural Products with Anti-Biofilm Activity. Curr. Org. Chem. 2018, 22, 789–817.
- Prabu, G.R.; Gnanamani, A.; Sadulla, S. Guaijaverin—A Plant Flavonoid as Potential Antiplaque Agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495.
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some Phytochemical, Pharmacological and Toxicological Properties of Ginger (Zingiber officinale Roscoe): A Review of Recent Research. Food Chem. Toxicol. 2008, 46, 409–420.
- Kim, H.-S.; Park, H.-D. Ginger Extract Inhibits Biofilm Formation by Pseudomonas aeruginosa PA14. PLoS ONE 2013, 8, e76106.
- Kumar, L.; Chhibber, S.; Harjai, K. Zingerone Inhibit Biofilm Formation and Improve Antibiofilm Efficacy of Ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia 2013, 90, 73–78.
- GBIF. Sistema Global de Información Sobre Biodiversidad. Available online: https://www.gbif.org/es/ (accessed on 25 July 2023).
- Cheek, M.; Nic Lughadha, E.; Kirk, P.; Lindon, H.; Carretero, J.; Looney, B.; Douglas, B.; Haelewaters, D.; Gaya, E.; Llewellyn, T.; et al. New Scientific Discoveries: Plants and Fungi. Plants People Planet 2020, 2, 371–388.
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194.
- Rokas, A. Evolution of the Human Pathogenic Lifestyle in Fungi. Nat. Microbiol. 2022, 7, 607–619.
- Restrepo-Rivera, L.M.; Cardona-Castro, N. Micobioma: Diversidad Fúngica En El Cuerpo Humano. CES Med. 2021, 35, 113–125.
- Seelbinder, B.; Chen, J.; Brunke, S.; Vazquez-Uribe, R.; Santhaman, R.; Meyer, A.C.; De Oliveira Lino, F.S.; Chan, K.F.; Loos, D.; Imamovic, L.; et al. Antibiotics Create a Shift from Mutualism to Competition in Human Gut Communities with a Longer-Lasting Impact on Fungi than Bacteria. Microbiome 2020, 8, 133.
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57.
- Guarro, J. Taxonomía y Biología de Los Hongos Causantes de Infección En Humanos. Enferm. Infecc. Microbiol. Clin. 2012, 30, 33–39.
- Köhler, J.R.; Casadevall, A.; Perfect, J. The Spectrum of Fungi That Infects Humans. Cold Spring Harb. Perspect. Med. 2015, 5, a019273.
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13.
- Pemán, J.; Salavert, M. Epidemiología General de La Enfermedad Fúngica Invasora. Enfermedades Infecc. Microbiol. Clínica 2012, 30, 90–98.
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267.
- Perfect, J.R. The Antifungal Pipeline: A Reality Check. Nat. Rev. Drug Discov. 2017, 16, 603–616.
- Oliveira, L.V.N.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for Human Fungal Diseases: Close but Still a Long Way to Go. Npj Vaccines 2021, 6, 33.
- Romani, L. Immunity to Fungal Infections. Nat. Rev. Immunol. 2011, 11, 275–288.
- Carmo, A.; Rocha, M.; Pereirinha, P.; Tomé, R.; Costa, E. Antifungals: From Pharmacokinetics to Clinical Practice. Antibiotics 2023, 12, 884.
- Quiles-Melero, I.; García-Rodríguez, J. Systemic Antifungal Drugs. Rev. Iberoam. Micol. 2021, 38, 42–46.
- Ruiz-Camps, I.; Cuenca-Estrella, M. Antifungals for Systemic Use. Enferm. Infecc. Microbiol. Clin. 2009, 27, 353–362.
- Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J. Fungi 2020, 6, 239.
- Gonzalez-Lara, M.F.; Sifuentes-Osornio, J.; Ostrosky-Zeichner, L. Drugs in Clinical Development for Fungal Infections. Drugs 2017, 77, 1505–1518.
- Hope, W.W.; McEntee, L.; Livermore, J.; Whalley, S.; Johnson, A.; Farrington, N.; Kolamunnage-Dona, R.; Schwartz, J.; Kennedy, A.; Law, D.; et al. Pharmacodynamics of the Orotomides against Aspergillus fumigatus: New Opportunities for Treatment of Multidrug-Resistant Fungal Disease. mBio 2017, 8, e01157-17.
- Fairlamb, A.H.; Gow, N.A.R.; Matthews, K.R.; Waters, A.P. Drug Resistance in Eukaryotic Microorganisms. Nat. Microbiol. 2016, 1, 16092.
- Berman, J.; Krysan, D.J. Drug Resistance and Tolerance in Fungi. Nat. Rev. Microbiol. 2020, 18, 319–331.
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571.
- Shrestha, S.K.; Fosso, M.Y.; Garneau-Tsodikova, S. A Combination Approach to Treating Fungal Infections. Sci. Rep. 2015, 5, 17070.
- Carpinella, M.C.; Andrione, D.G.; Ruiz, G.; Palacios, S.M. Screening for Acetylcholinesterase Inhibitory Activity in Plant Extracts from Argentina. Phytother. Res. 2010, 24, 259–263.
- Méndez-Chávez, M.; Ledesma-Escobar, C.A.; Hidalgo-Morales, M.; Rodríguez-Jimenes, G.D.C.; Robles-Olvera, V.J. Antifungal Activity Screening of Fractions from Annona cherimola Mill. Leaf Extract against Fusarium oxysporum. Arch. Microbiol. 2022, 204, 330.
- Campos, L.M.; Silva, T.P.; De Oliveira Lemos, A.S.; Mendonça Diniz, I.O.; Palazzi, C.; Novaes Da Rocha, V.; De Freitas Araújo, M.G.; Melo, R.C.N.; Fabri, R.L. Antibiofilm Potential of Annona muricata L. Ethanolic Extract against Multi-Drug Resistant Candida Albicans. J. Ethnopharmacol. 2023, 315, 116682.
- Diaz Napal, G.N.; Buffa, L.M.; Nolli, L.C.; Defagó, M.T.; Valladares, G.R.; Carpinella, M.C.; Ruiz, G.; Palacios, S.M. Screening of Native Plants from Central Argentina against the Leaf-Cutting Ant Acromyrmex lundi (Guérin) and Its Symbiotic Fungus. Ind. Crops Prod. 2015, 76, 275–280.
- Navarro García, V.M.; Gonzalez, A.; Fuentes, M.; Aviles, M.; Rios, M.Y.; Zepeda, G.; Rojas, M.G. Antifungal Activities of Nine Traditional Mexican Medicinal Plants. J. Ethnopharmacol. 2003, 87, 85–88.
- Poma-Castillo, L.; Espinoza-Poma, M. Antifungal Activity of Ethanol-Extracted Bixa Orellana (L) (Achiote) on Candida Albicans, at Six Different Concentrations. J. Contemp. Dent. Pract. 2019, 20, 1159–1163.
- Wilson, B.; Abraham, G.; Manju, V.S.; Mathew, M.; Vimala, B.; Sundaresan, S.; Nambisan, B. Antimicrobial Activity of Curcuma Zedoaria and Curcuma Malabarica Tubers. J. Ethnopharmacol. 2005, 99, 147–151.
- Chen, X.; Wang, C.; Xu, L.; Chen, X.; Wang, W.; Yang, G.; Tan, R.X.; Li, E.; Jin, Y. A Laboratory Evaluation of Medicinal Herbs Used in China for the Treatment of Hand, Foot, and Mouth Disease. Evid. Based Complement. Alternat. Med. 2013, 2013, 504563.
- Mir-Rashed, N.; Cruz, I.; Jessulat, M.; Dumontier, M.; Chesnais, C.; Juliana, N.; Amiguet, V.T.; Golshani, A.; Arnason, J.T.; Smith, M.L. Disruption of Fungal Cell Wall by Antifungal Echinacea Extracts. Med. Mycol. 2010, 48, 949–958.
- Merali, S.; Binns, S.; Paulin-Levasseur, M.; Ficker, C.; Smith, M.; Baum, B.; Brovelli, E.; Arnason, J.T. Antifungal and Anti-Inflammatory Activity of the Genus Echinacea. Pharm. Biol. 2003, 41, 412–420.
- Muschietti, L.; Derita, M.; Sülsen, V.; De Dios Muñoz, J.; Ferraro, G.; Zacchino, S.; Martino, V. In Vitro Antifungal Assay of Traditional Argentine Medicinal Plants. J. Ethnopharmacol. 2005, 102, 233–238.
- Fred-Jaiyesimi, A.A.; Abo, K.A. Phytochemical and Antimicrobial Analysis of the Crude Extract, Petroleum Ether and Chloroform Fractions of Euphorbia Heterophylla Linn Whole Plant. Pharmacogn. J. 2010, 2, 1–4.
- Sharma, A.; Angulo-Bejarano, P.; Madariaga-Navarrete, A.; Oza, G.; Iqbal, H.; Cardoso-Taketa, A.; Luisa Villarreal, M. Multidisciplinary Investigations on Galphimia Glauca: A Mexican Medicinal Plant with Pharmacological Potential. Molecules 2018, 23, 2985.
- Alshami, I.; Alharbi, A.E. Hibiscus sabdariffa Extract Inhibits in Vitro Biofilm Formation Capacity of Candida albicans Isolated from Recurrent Urinary Tract Infections. Asian Pac. J. Trop. Biomed. 2014, 4, 104–108.
- Quiroga, E.N.; Sampietro, A.R.; Vattuone, M.A. Screening Antifungal Activities of Selected Medicinal Plants. J. Ethnopharmacol. 2001, 74, 89–96.
- Moreno, M.A.; Córdoba, S.; Zampini, I.C.; Mercado, M.I.; Ponessa, G.; Sayago, J.E.; Ramos, L.L.P.; Schmeda-Hirschmann, G.; Isla, M.I. Argentinean Larrea Dry Extracts with Potential Use in Vaginal Candidiasis. Nat. Prod. Commun. 2018, 13, 1934578X1801300.
- Navarro-García, V.M.; Rojas, G.; Avilés, M.; Fuentes, M.; Zepeda, G. In Vitro Antifungal Activity of Coumarin Extracted from Loeselia Mexicana Brand: Antifungal Coumarins from Loeselia mexicana. Mycoses 2011, 54, e569–e571.
- Morais-Braga, M.F.B.; Souza, T.M.; Santos, K.K.A.; Andrade, J.C.; Guedes, G.M.M.; Tintino, S.R.; Sobral-Souza, C.E.; Costa, J.G.M.; Menezes, I.R.A.; Saraiva, A.A.F.; et al. Antimicrobial and Modulatory Activity of Ethanol Extract of the Leaves from Lygodium venustum SW. Am. Fern J. 2012, 102, 154–160.
- Navarro García, V.M.; Rojas, G.; Gerardo Zepeda, L.; Aviles, M.; Fuentes, M.; Herrera, A.; Jiménez, E. Antifungal and Antibacterial Activity of Four Selected Mexican Medicinal Plants. Pharm. Biol. 2006, 44, 297–300.
- AL-Rubaey, N.K.F.; Abbas, F.M.; Hameed, I.H. Antibacterial and Anti-Fungal Activity of Methanolic Extract of Passiflora caerulea. Indian J. Public Health Res. Dev. 2019, 10, 930.
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal Effect of Pimenta Dioica Essential Oil against Dangerous Pathogenic and Toxinogenic Fungi. Ind. Crops Prod. 2009, 30, 250–253.
- Derita, M.G.; Leiva, M.L.; Zacchino, S.A. Influence of Plant Part, Season of Collection and Content of the Main Active Constituent, on the Antifungal Properties of Polygonum Acuminatum Kunth. J. Ethnopharmacol. 2009, 124, 377–383.
- Javed, B.; Farooq, F.; Ibrahim, M.; Abbas, H.A.B.; Jawwad, H.; Zehra, S.S.; Ahmad, H.M.; Sarwer, A.; Malik, K.; Nawaz, K. Antibacterial and Antifungal Activity of Methanolic Extracts of Salix alba L. against Various Disease Causing Pathogens. Braz. J. Biol. 2023, 83, e243332.
- Hnatyszyn, O.; Juárez, S.; Ouviña, A.; Martino, V.; Zacchino, S.; Ferraro, G. Phytochemical Analysis and Antifungal Evaluation of Sebastiania commersoniana. Extracts. Pharm. Biol. 2007, 45, 404–406.
- Cáceres, A.; Cruz, S.M.; Martínez, V.; Gaitán, I.; Santizo, A.; Gattuso, S.; Gattuso, M. Ethnobotanical, Pharmacognostical, Pharmacological and Phytochemical Studies on Smilax Domingensis in Guatemala. Rev. Bras. Farmacogn. 2012, 22, 239–248.
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal Activity of the Clove Essential Oil from Syzygium Aromaticum on Candida, Aspergillus and Dermatophyte Species. J. Med. Microbiol. 2009, 58, 1454–1462.
- Awere, C.A.; Githae, E.W.; Gichumbi, J.M. Phytochemical Analysis and Antifungal Activity of Tithonia diversifolia and Kigelia africana Extracts against Fusarium Oxysporum in Tomato. Afr. J. Agric. Res. 2021, 17, 726–732.
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. against Human Pathogens. Molecules 2020, 25, 3748.
- Svetaz, L.; Agüero, M.; Alvarez, S.; Luna, L.; Feresin, G.; Derita, M.; Tapia, A.; Zacchino, S. Antifungal Activity of Zuccagnia Punctata Cav.: Evidence for the Mechanism of Action. Planta Med. 2007, 73, 1074–1080.
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus Taxonomy: The Database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717.
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367.
- Cliver, D.O. Capsid and Infectivity in Virus Detection. Food Environ. Virol. 2009, 1, 123–128.
- Jassim, S.A.; Naji, M.A. Novel Antiviral Agents: A Medicinal Plant Perspective. J. Appl. Microbiol. 2003, 95, 412–427.
- Sharma, N. Efficacy of Garlic and Onion against Virus. Int. J. Res. Pharm. Sci. 2019, 10, 3578–3586.
- Hudson, J.; Vimalanathan, S. Echinacea—A Source of Potent Antivirals for Respiratory Virus Infections. Pharmaceuticals 2011, 4, 1019–1031.
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A. In Vitro Virucidal Activity of Echinaforce®, an Echinacea purpurea Preparation, against Coronaviruses, Including Common Cold Coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136.
- Weng, J.-R.; Lin, C.-S.; Lai, H.-C.; Lin, Y.-P.; Wang, C.-Y.; Tsai, Y.-C.; Wu, K.-C.; Huang, S.-H.; Lin, C.-W. Antiviral Activity of Sambucus Formosana Nakai Ethanol Extract and Related Phenolic Acid Constituents against Human Coronavirus NL63. Virus Res. 2019, 273, 197767.
- Chathuranga, K.; Kim, M.S.; Lee, H.-C.; Kim, T.-H.; Kim, J.-H.; Gayan Chathuranga, W.A.; Ekanayaka, P.; Wijerathne, H.; Cho, W.-K.; Kim, H.I. Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts in Vitro and in Vivo. Viruses 2019, 11, 604.
- Kunsorn, P.; Ruangrungsi, N.; Lipipun, V.; Khanboon, A.; Rungsihirunrat, K. The Identities and Anti-Herpes Simplex Virus Activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac. J. Trop. Biomed. 2013, 3, 284–290.
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral Activity of Plantago major Extracts and Related Compounds in Vitro. Antivir. Res. 2002, 55, 53–62.
- Wahab, N.Z.A.; Bunawan, H.; Ibrahim, N. Cytotoxicity and Antiviral Activity of Methanol Extract from Polygonum minus. In Proceedings of the AIP Conference Proceedings, Selangor, Malaysia, 15–16 April 2015; AIP Publishing: Melville, NY, USA, 2015; Volume 1678.
- Zandi, K.; Zadeh, M.A.; Sartavi, K.; Rastian, Z. Antiviral Activity of Aloe vera against Herpes Simplex Virus Type 2: An in Vitro Study. Afr. J. Biotechnol. 2007, 6, 1770–1773.
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources Against Herpes Simplex Viruses. Viruses 2021, 13, 1386.
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral Activity of the Lippia graveolens (Mexican Oregano) Essential Oil and Its Main Compound Carvacrol against Human and Animal Viruses. Braz. J. Microbiol. 2011, 42, 1616–1624.
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral Effect of Compounds Derived from Angelica archangelica L. on Herpes Simplex Virus-1 and Coxsackievirus B3 Infections. Food Chem. Toxicol. 2017, 109, 1026–1031.
- Kurokawa, M.; Hozumi, T.; Basnet, P.; Nakano, M.; Kadota, S.; Namba, T.; Kawana, T.; Shiraki, K. Purification and Characterization of Eugeniin as an Anti-Herpesvirus Compound from Geum Japonicum Andsyzygium Aromaticum. J. Pharmacol. Exp. Ther. 1998, 284, 728–735.
- Schnitzler, P.; Schuhmacher, A.; Astani, A.; Reichling, J. Melissa officinalis Oil Affects Infectivity of Enveloped Herpesviruses. Phytomedicine 2008, 15, 734–740.
- Mazzanti, G.; Battinelli, L.; Pompeo, C.; Serrilli, A.M.; Rossi, R.; Sauzullo, I.; Mengoni, F.; Vullo, V. Inhibitory Activity of Melissa officinalis L. Extract on Herpes Simplex Virus Type 2 Replication. Nat. Prod. Res. 2008, 22, 1433–1440.
- Astani, A.; Heidary Navid, M.; Schnitzler, P. Attachment and Penetration of Acyclovir-Resistant Herpes Simplex Virus Are Inhibited by Melissa officinalis Extract. Phytother. Res. 2014, 28, 1547–1552.
- Liu, S.; Li, H.; Chen, L.; Yang, L.; Li, L.; Tao, Y.; Li, W.; Li, Z.; Liu, H.; Tang, M. (-)-Epigallocatechin-3-Gallate Inhibition of Epstein–Barr Virus Spontaneous Lytic Infection Involves ERK1/2 and PI3-K/Akt Signaling in EBV-Positive Cells. Carcinogenesis 2013, 34, 627–637.
- Kapadia, G.J.; Azuine, M.A.; Tokuda, H.; Takasaki, M.; Mukainaka, T.; Konoshima, T.; Nishino, H. Chemopreventive Effect of Resveratrol, Sesamol, Sesame Oil and Sunflower Oil in the Epstein–Barr Virus Early Antigen Activation Assay and the Mouse Skin Two-Stage Carcinogenesis. Pharmacol. Res. 2002, 45, 499–505.
- Konoshima, T.; Takasaki, M.; Kozuka, M.; Tokuda, H.; Nishino, H.; Iwashima, A.; Haruna, M.; Ito, K.; Tanabe, M. Inhibitory Effects on Epstein-Barr Virus Activation and Anti-Tumor Promoting Activities of Neolignans from Magnolia officinalis. Planta Med. 1990, 56, 653.
- Wang, C.; Wang, H.; Zhang, Y.; Guo, W.; Long, C.; Wang, J.; Liu, L.; Sun, X. Berberine Inhibits the Proliferation of Human Nasopharyngeal Carcinoma Cells via an Epstein-Barr Virus Nuclear Antigen 1-Dependent Mechanism. Oncol. Rep. 2017, 37, 2109–2120.
- Hergenhahn, M.; Soto, U.; Weninger, A.; Polack, A.; Hsu, C.-H.; Cheng, A.-L.; Rösl, F. The Chemopreventive Compound Curcumin Is an Efficient Inhibitor of Epstein-Barr Virus BZLF1 Transcription in Raji DR-LUC Cells. Mol. Carcinog. 2002, 33, 137–145.
- Wu, C.-C.; Fang, C.-Y.; Cheng, Y.-J.; Hsu, H.-Y.; Chou, S.-P.; Huang, S.-Y.; Tsai, C.-H.; Chen, J.-Y. Inhibition of Epstein-Barr Virus Reactivation by the Flavonoid Apigenin. J. Biomed. Sci. 2017, 24, 2.
- Lin, J.-C.; Cherng, J.-M.; Hung, M.-S.; Baltina, L.A.; Baltina, L.; Kondratenko, R. Inhibitory Effects of Some Derivatives of Glycyrrhizic Acid against Epstein-Barr Virus Infection: Structure–Activity Relationships. Antivir. Res. 2008, 79, 6–11.
- Wu, C.-C.; Fang, C.-Y.; Hsu, H.-Y.; Chen, Y.-J.; Chou, S.-P.; Huang, S.-Y.; Cheng, Y.-J.; Lin, S.-F.; Chang, Y.; Tsai, C.-H. Luteolin Inhibits Epstein-Barr Virus Lytic Reactivation by Repressing the Promoter Activities of Immediate-Early Genes. Antivir. Res. 2016, 132, 99–110.
- Evers, D.L.; Chao, C.-F.; Wang, X.; Zhang, Z.; Huong, S.-M.; Huang, E.-S. Human Cytomegalovirus-Inhibitory Flavonoids: Studies on Antiviral Activity and Mechanism of Action. Antivir. Res. 2005, 68, 124–134.
- Wang, S.-Y.; Zhang, J.; Xu, X.-G.; Su, H.-L.; Xing, W.-M.; Zhang, Z.-S.; Jin, W.-H.; Dai, J.-H.; Wang, Y.-Z.; He, X.-Y. Inhibitory Effects of Piceatannol on Human Cytomegalovirus (hCMV) in Vitro. J. Microbiol. 2020, 58, 716–723.
- Evers, D.L.; Wang, X.; Huong, S.-M.; Huang, D.Y.; Huang, E.-S. 3,4′,5-Trihydroxy-Trans-Stilbene (Resveratrol) Inhibits Human Cytomegalovirus Replication and Virus-Induced Cellular Signaling. Antivir. Res. 2004, 63, 85–95.
- Zhen, H.; Fang, F.; Ye, D.; Shu, S.; Zhou, Y.; Dong, Y.; Nie, X.; Li, G. Experimental Study on the Action of Allitridin against Human Cytomegalovirus in Vitro: Inhibitory Effects on Immediate-Early Genes. Antivir. Res. 2006, 72, 68–74.
- Abd-Elazem, I.S.; Chen, H.S.; Bates, R.B.; Huang, R.C.C. Isolation of Two Highly Potent and Non-Toxic Inhibitors of Human Immunodeficiency Virus Type 1 (HIV-1) Integrase from Salvia miltiorrhiza. Antivir. Res. 2002, 55, 91–106.
- Locher, C.P.; Witvrouw, M.; De Béthune, M.P.; Burch, M.T.; Mower, H.F.; Davis, H.; Lasure, A.; Pauwels, R.; De Clercq, E.; Vlietinck, A.J. Antiviral Activity of Hawaiian Medicinal Plants against Human Immunodeficiency Virus Type-1 (HIV-1). Phytomed. Int. J. Phytother. Phytopharm. 1996, 2, 259–264.
- Nutan; Modi, M.; Dezzutti, C.S.; Kulshreshtha, S.; Rawat, A.K.S.; Srivastava, S.K.; Malhotra, S.; Verma, A.; Ranga, U.; Gupta, S.K. Extracts from Acacia catechu Suppress HIV-1 Replication by Inhibiting the Activities of the Viral Protease and Tat. Virol. J. 2013, 10, 309.
- Notka, F.; Meier, G.; Wagner, R. Concerted Inhibitory Activities of Phyllanthus Amarus on HIV Replication in Vitro and Ex Vivo. Antivir. Res. 2004, 64, 93–102.
- Lee-Huang, S.; Zhang, L.; Huang, P.L.; Chang, Y.-T.; Huang, P.L. Anti-HIV Activity of Olive Leaf Extract (OLE) and Modulation of Host Cell Gene Expression by HIV-1 Infection and OLE Treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037.
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral Potentials of Medicinal Plants. Virus Res. 2008, 131, 111–120.
- Bessong, P.O.; Obi, C.L.; Andréola, M.-L.; Rojas, L.B.; Pouységu, L.; Igumbor, E.; Meyer, J.J.M.; Quideau, S.; Litvak, S. Evaluation of Selected South African Medicinal Plants for Inhibitory Properties against Human Immunodeficiency Virus Type 1 Reverse Transcriptase and Integrase. J. Ethnopharmacol. 2005, 99, 83–91.
- Zhang, X.; Yang, L.-M.; Liu, G.-M.; Liu, Y.-J.; Zheng, C.-B.; Lv, Y.-J.; Li, H.-Z.; Zheng, Y.-T. Potent Anti-HIV Activities and Mechanisms of Action of a Pine Cone Extract from Pinus yunnanensis. Molecules 2012, 17, 6916–6929.
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV Activity of Extracts from Calendula officinalis Flowers. Biomed. Pharmacother. 1997, 51, 176–180.
- Callies, O.; Bedoya, L.M.; Beltrán, M.; Munoz, A.; Calderón, P.O.; Osorio, A.A.; Jiménez, I.A.; Alcami, J.; Bazzocchi, I.L. Isolation, Structural Modification, and HIV Inhibition of Pentacyclic Lupane-Type Triterpenoids from Cassine xylocarpa and Maytenus cuzcoina. J. Nat. Prod. 2015, 78, 1045–1055.
- Hao, B.-J.; Wu, Y.-H.; Wang, J.-G.; Hu, S.-Q.; Keil, D.J.; Hu, H.-J.; Lou, J.-D.; Zhao, Y. Hepatoprotective and Antiviral Properties of Isochlorogenic Acid A from Laggera alata against Hepatitis B Virus Infection. J. Ethnopharmacol. 2012, 144, 190–194.
- Jiang, Z.-Y.; Liu, W.-F.; Zhang, X.-M.; Luo, J.; Ma, Y.-B.; Chen, J.-J. Anti-HBV Active Constituents from Piper longum. Bioorg. Med. Chem. Lett. 2013, 23, 2123–2127.
- Zeng, F.-L.; Xiang, Y.-F.; Liang, Z.-R.; Wang, X.; Huang, D.-E.; Zhu, S.-N.; Li, M.-M.; Yang, D.-P.; Wang, D.-M.; Wang, Y.-F. Anti-Hepatitis B Virus Effects of Dehydrocheilanthifoline from Corydalis saxicola. Am. J. Chin. Med. 2013, 41, 119–130.
- Chiang, L.-C.; Ng, L.T.; Liu, L.-T.; Shieh, D.-E.; Lin, C.-C. Cytotoxicity and Anti-Hepatitis B Virus Activities of Saikosaponins from Bupleurum Species. Planta Med. 2003, 69, 705–709.
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816.
- Rechtman, M.M.; Har-Noy, O.; Bar-Yishay, I.; Fishman, S.; Adamovich, Y.; Shaul, Y.; Halpern, Z.; Shlomai, A. Curcumin Inhibits Hepatitis B Virus via Down-Regulation of the Metabolic Coactivator PGC-1alpha. FEBS Lett. 2010, 584, 2485–2490.
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal Plants: Treasure for Antiviral Drug Discovery. Phytother. Res. PTR 2021, 35, 3447–3483.
- Karamese, M.; Aydogdu, S.; Karamese, S.A.; Altoparlak, U.; Gundogdu, C. Preventive Effects of a Major Component of Green Tea, Epigallocathechin-3-Gallate, on Hepatitis-B Virus DNA Replication. Asian Pac. J. Cancer Prev. 2015, 16, 4199–4202.
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.W.; Liu, Y.; Graf, T.N.; Oberlies, N.H. Identification of Hepatoprotective Flavonolignans from Silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999.
- Kim, K.; Kim, K.H.; Kim, H.Y.; Cho, H.K.; Sakamoto, N.; Cheong, J. Curcumin Inhibits Hepatitis C Virus Replication via Suppressing the Akt-SREBP-1 Pathway. FEBS Lett. 2010, 584, 707–712.
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouillé, Y.; Séron, K. (-)-Epigallocatechin-3-Gallate Is a New Inhibitor of Hepatitis C Virus Entry. Hepatology 2012, 55, 720–729.
- Haid, S.; Novodomská, A.; Gentzsch, J.; Grethe, C.; Geuenich, S.; Bankwitz, D.; Chhatwal, P.; Jannack, B.; Hennebelle, T.; Bailleul, F.; et al. A Plant-Derived Flavonoid Inhibits Entry of All HCV Genotypes into Human Hepatocytes. Gastroenterology 2012, 143, 213–222.e5.
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin Has Antiviral Activity against Hepatitis C Virus. Antimicrob. Agents Chemother. 2011, 55, 5159–5167.
- Lin, L.-T.; Chen, T.-Y.; Lin, S.-C.; Chung, C.-Y.; Lin, T.-C.; Wang, G.-H.; Anderson, R.; Lin, C.-C.; Richardson, C.D. Broad-Spectrum Antiviral Activity of Chebulagic Acid and Punicalagin against Viruses That Use Glycosaminoglycans for Entry. BMC Microbiol. 2013, 13, 187.
- Wahyuni, T.S.; Widyawaruyanti, A.; Lusida, M.I.; Fuad, A.; Soetjipto; Fuchino, H.; Kawahara, N.; Hayashi, Y.; Aoki, C.; Hotta, H. Inhibition of Hepatitis C Virus Replication by Chalepin and Pseudane IX Isolated from Ruta angustifolia Leaves. Fitoterapia 2014, 99, 276–283.
- Shikov, A.N.; Djachuk, G.I.; Sergeev, D.V.; Pozharitskaya, O.N.; Esaulenko, E.V.; Kosman, V.M.; Makarov, V.G. Birch Bark Extract as Therapy for Chronic Hepatitis C–a Pilot Study. Phytomedicine 2011, 18, 807–810.
- Moradi, M.-T.; Karimi, A.; Shahrani, M.; Hashemi, L.; Ghaffari-Goosheh, M.-S. Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions. Avicenna J. Med. Biotechnol. 2019, 11, 285–291.
- Pantev, A.; Ivancheva, S.; Staneva, L.; Serkedjieva, J. Biologically Active Constituents of a Polyphenol Extract from Geranium sanguineum L. with Anti-Influenza Activity. Z. Naturforschung C J. Biosci. 2006, 61, 508–516.
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Ogbomo, H.; Altenbrandt, B.; Leutz, A.; Doerr, H.W.; Cinatl, J. Glycyrrhizin Inhibits Highly Pathogenic H5N1 Influenza A Virus-Induced pro-Inflammatory Cytokine and Chemokine Expression in Human Macrophages. Med. Microbiol. Immunol. 2010, 199, 291–297.
- Sawai, R.; Kuroda, K.; Shibata, T.; Gomyou, R.; Osawa, K.; Shimizu, K. Anti-Influenza Virus Activity of Chaenomeles sinensis. J. Ethnopharmacol. 2008, 118, 108–112.
- Zakay-Rones, Z.; Varsano, N.; Zlotnik, M.; Manor, O.; Regev, L.; Schlesinger, M.; Mumcuoglu, M. Inhibition of Several Strains of Influenza Virus in Vitro and Reduction of Symptoms by an Elderberry Extract (Sambucus nigra L.) during an Outbreak of Influenza B Panama. J. Altern. Complement. Med. 1995, 1, 361–369.
- Liu, G.; Xiong, S.; Xiang, Y.-F.; Guo, C.-W.; Ge, F.; Yang, C.-R.; Zhang, Y.-J.; Wang, Y.-F.; Kitazato, K. Antiviral Activity and Possible Mechanisms of Action of Pentagalloylglucose (PGG) against Influenza A Virus. Arch. Virol. 2011, 156, 1359–1369.
- Song, J.-M.; Lee, K.-H.; Seong, B.-L. Antiviral Effect of Catechins in Green Tea on Influenza Virus. Antivir. Res. 2005, 68, 66–74.
- Hayashi, K.; Narutaki, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Therapeutic Effect of Arctiin and Arctigenin in Immunocompetent and Immunocompromised Mice Infected with Influenza A Virus. Biol. Pharm. Bull. 2010, 33, 1199–1205.
- Schapowal, A. Efficacy and Safety of Echinaforce® in Respiratory Tract Infections. Wien. Med. Wochenschr. 1946 2013, 163, 102–105.
- Dall’Acqua, S.; Perissutti, B.; Grabnar, I.; Farra, R.; Comar, M.; Agostinis, C.; Caristi, G.; Golob, S.; Voinovich, D. Pharmacokinetics and Immunomodulatory Effect of Lipophilic Echinacea Extract Formulated in Softgel Capsules. Eur. J. Pharm. Biopharm. 2015, 97, 8–14.
- Rauš, K.; Pleschka, S.; Klein, P.; Schoop, R.; Fisher, P. Effect of an Echinacea-Based Hot Drink versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial. Curr. Ther. Res. 2015, 77, 66–72.
- Pourghanbari, G.; Nili, H.; Moattari, A.; Mohammadi, A.; Iraji, A. Antiviral Activity of the Oseltamivir and Melissa officinalis L. Essential Oil against Avian Influenza A Virus (H9N2). Virus Dis. 2016, 27, 170–178.
- Wyde, P.R.; Meyerson, L.R.; Gilbert, B.E. In Vitro Evaluation of the Antiviral Activity of SP-303, an Euphorbiaceae Shrub Extract, against a Panel of Respiratory Viruses. Drug Dev. Res. 1993, 28, 467–472.
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.-H.S.; Garcia, F. Green Tea Compound in Chemoprevention of Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2010, 20, 617–624.
- Mondal, A.; Chatterji, U. Artemisinin Represses Telomerase Subunits and Induces Apoptosis in HPV-39 Infected Human Cervical Cancer Cells. J. Cell. Biochem. 2015, 116, 1968–1981.
- Yarnell, E. Herbs against Human Papillomavirus. Altern. Complement. Ther. 2015, 21, 71–76.
- Jiang, W.-L.; Luo, X.-L.; Kuang, S.-J. Effects of Alternanthera philoxeroides Griseb against dengue virus in vitro. 1 Jun Yi Xue Xue Bao Acad. J. First Med. Coll. PLA 2005, 25, 454–456.
- Salim, F.; Abu, N.A.; Yaakob, H.; Kadir, L.; Zainol, N.; Taher, Z. Interaction of Carica papaya L. Leaves Optimum Extract on Virus Dengue Infected Cells. Sci. Int. 2018, 30, 437–441.
- Castillo-Maldonado, I.; Moreno-Altamirano, M.M.B.; Serrano-Gallardo, L.B. Anti-Dengue Serotype-2 Activity Effect of Sambucus nigra Leaves-and Flowers-Derived Compounds. Virol. Res. Rev. 2017, 1, 1–5.
- Chan, Y.S.; Khoo, K.S.; Sit, N.W.W. Investigation of Twenty Selected Medicinal Plants from Malaysia for Anti-Chikungunya Virus Activity. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2016, 19, 175–182.
- Raghavendhar, S.; Tripati, P.K.; Ray, P.; Patel, A.K. Evaluation of Medicinal Herbs for Anti-CHIKV Activity. Virology 2019, 533, 45–49.
- Li, S.-Y.; Chen, C.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-G.; et al. Identification of Natural Compounds with Antiviral Activities against SARS-Associated Coronavirus. Antivir. Res. 2005, 67, 18–23.
- Ho, T.-Y.; Wu, S.-L.; Chen, J.-C.; Li, C.-C.; Hsiang, C.-Y. Emodin Blocks the SARS Coronavirus Spike Protein and Angiotensin-Converting Enzyme 2 Interaction. Antivir. Res. 2007, 74, 92–101.
- Wen, C.-C.; Shyur, L.-F.; Jan, J.-T.; Liang, P.-H.; Kuo, C.-J.; Arulselvan, P.; Wu, J.-B.; Kuo, S.-C.; Yang, N.-S. Traditional Chinese Medicine Herbal Extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis Inhibit SARS-CoV Replication. J. Tradit. Complement. Med. 2011, 1, 41–50.
- Ulasli, M.; Gurses, S.A.; Bayraktar, R.; Yumrutas, O.; Oztuzcu, S.; Igci, M.; Igci, Y.Z.; Cakmak, E.A.; Arslan, A. The Effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) Extracts on the Replication of Coronavirus and the Expression of TRP Genes Family. Mol. Biol. Rep. 2014, 41, 1703–1711.
- Lin, C.-W.; Tsai, F.-J.; Tsai, C.-H.; Lai, C.-C.; Wan, L.; Ho, T.-Y.; Hsieh, C.-C.; Chao, P.-D.L. Anti-SARS Coronavirus 3C-like Protease Effects of Isatis Indigotica Root and Plant-Derived Phenolic Compounds. Antivir. Res. 2005, 68, 36–42.
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Nguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya Nucifera Displaying SARS-CoV 3CL(pro) Inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947.
- Remali, J.; Aizat, W.M. A Review on Plant Bioactive Compounds and Their Modes of Action Against Coronavirus Infection. Front. Pharmacol. 2020, 11, 589044.
- Huang, T.-J.; Tsai, Y.-C.; Chiang, S.-Y.; Wang, G.-J.; Kuo, Y.-C.; Chang, Y.-C.; Wu, Y.-Y.; Wu, Y.-C. Anti-Viral Effect of a Compound Isolated from Liriope platyphylla against Hepatitis B Virus in Vitro. Virus Res. 2014, 192, 16–24.
- Gansukh, E.; Kazibwe, Z.; Pandurangan, M.; Judy, G.; Kim, D.H. Probing the Impact of Quercetin-7-O-Glucoside on Influenza Virus Replication Influence. Phytomedicine 2016, 23, 958–967.
- Akher, F.B.; Farrokhzadeh, A.; Ramharack, P.; Shunmugam, L.; Van Heerden, F.R.; Soliman, M.E.S. Discovery of Novel Natural Flavonoids as Potent Antiviral Candidates against Hepatitis C Virus NS5B Polymerase. Med. Hypotheses 2019, 132, 109359.
- Sajitha Lulu, S.; Thabitha, A.; Vino, S.; Mohana Priya, A.; Rout, M. Naringenin and Quercetin—Potential Anti-HCV Agents for NS2 Protease Targets. Nat. Prod. Res. 2016, 30, 464–468.
- Moharana, M.; Pattanayak, S.K.; Khan, F. Molecular Recognition of Bio-Active Triterpenoids from Swertia chirayita towards Hepatitis Delta Antigen: A Mechanism through Docking, Dynamics Simulation, Gibbs Free Energy Landscape. J. Biomol. Struct. Dyn. 2023, 1–14.
- Shadrack, D.M.; Deogratias, G.; Kiruri, L.W.; Onoka, I.; Vianney, J.-M.; Swai, H.; Nyandoro, S.S. Luteolin: A Blocker of SARS-CoV-2 Cell Entry Based on Relaxed Complex Scheme, Molecular Dynamics Simulation, and Metadynamics. J. Mol. Model. 2021, 27, 221.
- Abdelli, I.; Hassani, F.; Bekkel Brikci, S.; Ghalem, S. In Silico Study the Inhibition of Angiotensin Converting Enzyme 2 Receptor of COVID-19 by Ammoides verticillata Components Harvested from Western Algeria. J. Biomol. Struct. Dyn. 2021, 39, 3263–3276.
- Kumar Verma, A.; Kumar, V.; Singh, S.; Goswami, B.C.; Camps, I.; Sekar, A.; Yoon, S.; Lee, K.W. Repurposing Potential of Ayurvedic Medicinal Plants Derived Active Principles against SARS-CoV-2 Associated Target Proteins Revealed by Molecular Docking, Molecular Dynamics and MM-PBSA Studies. Biomed. Pharmacother. 2021, 137, 111356.
- Rudrapal, M.; Issahaku, A.R.; Agoni, C.; Bendale, A.R.; Nagar, A.; Soliman, M.E.S.; Lokwani, D. In Silico Screening of Phytopolyphenolics for the Identification of Bioactive Compounds as Novel Protease Inhibitors Effective against SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 10437–10453.
This entry is offline, you can click here to edit this entry!