Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
The salinization of soils is a growing agricultural concern worldwide. Irrigation practices, drought, and climate change are leading to elevated salinity levels in many regions, resulting in reduced crop yields. However, there is potential for a solution in the microbiome of halophytes, which are naturally salt-tolerant plants. These plants harbor a salt-tolerant microbiome in their rhizosphere (around roots) and endosphere (within plant tissue). These bacteria may play a significant role in conferring salt tolerance to the host plants.
- salt tolerance
- halophilic bacteria
- Halomonas
- Kushneria
1. The Relationship between Bacteria and Plants in the Rhizosphere
The rhizosphere is the area in soil that immediately surrounds the roots of plants [36,37]. The rhizosphere contains countless species and a vast diversity of microorganisms [36,38]. One important component of the rhizosphere is plant mucilage. Mucilage is excreted by plant root tissue and can serve as a carbon source for microorganisms. The amount and composition of the mucilage can have a large impact on the bacterial species that live in the rhizosphere [37,39,40]. By excreting mucilage, plants attract beneficial microbes to the rhizosphere and benefit from microbial ability to break down sugars, fix nitrogen, suppress pathogens, etc. Mucilage can also play a role in attracting and supplying sugars for halophilic bacteria. These halophilic rhizobacteria could then, in turn, aid plants in saline soils. Bacteria found to be associated with the roots and that stimulate plant growth are termed plant-growth-promoting rhizobacteria (PGPR), and along with those that become established within plant tissue as endophytes are more broadly termed PGPB (plant-growth-promoting bacteria).
Species of bacteria and fungi that live within plant tissues without causing harm to the plant are defined as bacterial and fungal endophytes, respectively. There has been increasing interest in the role of endophytes in plant adaptation to different adverse environmental conditions in a variety of ways, as outlined in Figure 1 [41,42]. It has been well-established that microorganisms associated with plants thriving under harsh environmental niches play a crucial role in their adaptations to these suboptimal conditions [43]. The key mechanisms involved in such microbial-mediated adaptations to different stresses include modulation of phytohormones biosynthesis (auxins, cytokinins, gibberellins, abscisic acid, ACC deaminase (1-aminocyclopropane-1-carboxylate deaminase), brassinosteroids, and ethylene), accumulation of osmoprotectants (betaines, proline, and soluble sugars), upregulation of different defense genes, and production of secondary metabolites [43,44].
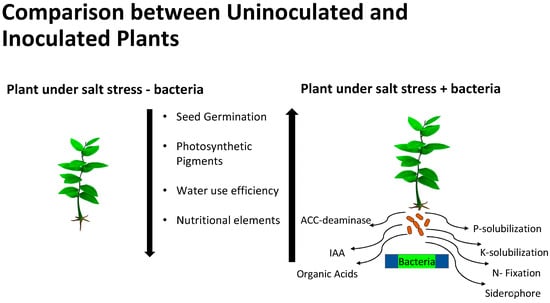
Figure 1. Effects of salt stress on plants and potential mechanisms of PGPR effects on plant growth in salty soils.
Plant hormones are chemical messengers that regulate various physiological processes in plants, including plant growth, development, and response to environmental stimuli such as light, temperature, and stress [45,46]. These hormones are master regulators, enabling plants to adapt and thrive in changing environmental conditions [47,48]. The diversity of phytohormones mirrors the multifaceted challenges that plants encounter in their environments. Each type of hormone is specialized in governing specific physiological responses, yet their interplay creates a sophisticated web of regulations that facilitate seamless coordination between different cellular and developmental processes. Moreover, plants often exhibit pleiotropy, where a single phytohormone can have widespread effects, influencing various aspects of plant life, while multiple hormones collaborate to finetune a particular biological event. This intricately woven system of phytohormonal control empowers plants to maintain homeostasis and optimize their growth in response to internal and external cues. In response to environmental changes, such as light, temperature, water availability, or the presence of pests and pathogens, plants dynamically adjust their phytohormone profiles. Studies have shown that in the presence of high concentrations of salt, production of auxin is severely limited. Plants grown in salty soils had insufficient auxin levels and suffered from stunted growth [48]. Different bacterial strains have been shown to induce hormone production and supplement those produced by the plant through the function of microRNAs (miRNAs) [47,49]. If a plant has insufficient hormone levels, the growth and development of the plant, as well as the fruit, can suffer and yield can be significantly decreased.
Indole-3-acetic acid (IAA) is a plant hormone that regulates the production of new root and shoot tissues. It has been shown that IAA produced by bacteria can induce adventitious shoot growth [44,47,50,51,52]. High salinity induces the utilization of 1-aminocyclopropane-1-carboxylic acid (ACC). ACC is a precursor for ethylene, a plant hormone that mediates a wide range of essential plant responses. However, at elevated levels, ethylene has a deleterious effect on root and shoot elongation, leaf expansion, and overall plant health [52]. ACC deaminase is an enzyme that breaks down ACC, preventing the production of ethylene, and it can alleviate the stress response in plants. ACC deaminase-producing bacteria can help aid the plant when it is under salt stress, and even help promote plant growth and antioxidant production [52], as has been shown for the French bean [53].
Microorganisms are known to produce over 20,000 different secondary metabolites [54]. These metabolites can affect the survival and performance of other organisms. Not only do endophytes produce secondary metabolites beneficial to plant growth and disease response but endophytes also produce novel biomolecules and plant growth promotors [55]. Because of these beneficial effects, the utilization of these endophytes holds potential to improve plant growth, particularly under adverse environmental conditions such as on marginal lands, and it may confer resilience to climate change.
2. Halophilic Bacteria with PGPB Potential
There are a considerable number of Bacillus strains that have been identified as having general PGPB activity (reviewed in [56]). To date, there has been some limited analysis of possible functions as ST-PGPB for some, which is briefly summarized here. One recent report suggests that a soil Bacillus strain is recruited to coastal halophytes by exudates from plant roots, but this work does not include plant growth stimulation studies [57]. There is also an interesting recent publication on combinations of a Bacillus strain with other bacteria including a Pseudomonas strain to promote plant growth [58]. Other work has shown the promise of a combination of bacterial and mycorrhizal fungal applications in enhancing soil fertility and rice production due to enhanced mineral uptake from the soil [59].
The microbial community found to be associated with halophytes growing in saline soils represents a rich source of ST-PGPB (salt-tolerant, halophilic, rhizobacteria, or endophytes) [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74]. Improvement of soil health and potential in bioremediation have also been reported [48,61]. A combination of strains may provide synergistic benefits. For example, one recent study showed the value of using a consortium of multiple halophilic strains isolated from halophytes to improve the growth of several crops under saline conditions [75]. Other studies have shown beneficial effects of ST-PGPB in reducing salt stress and improving yield in rice [76,77]. PGPB strains that have ACC deaminase and ROS scavenging activity have been shown to contribute to amelioration of salinity stress [78]. These reports provide a strong foundation for the use of halophiles isolated from the rhizosphere or roots of halophytes as inocula to stimulate the growth of salt-sensitive crops [79,80]. Recently, an Actinomycete was found to have antifungal activity and alleviate salt stress in tomato [81]. Another Actinomycete was reported to promote the growth of a halophyte using irrigation with seawater. Some biofilm-forming strains of Bacillus have been shown to enhance the growth of maize in saline conditions [82,83]. Another example is Bacillus subtilis (GB03), which was identified as a rhizosphere bacterium that stimulates growth of white clover under saline conditions [84], and which has since been renamed Bacillus amyloliquifaciens GB03 [85,86]. This strain produces volatile compounds that enhance plant photosynthetic capacity and chlorophyll content and induces an elevation of endogenous sugar content and suppression of abscisic acid (ABA)-induced RNA transcripts. Some of these strains also aid in dealing with other types of abiotic stress [87]. B. amyloliquifaciences GB03 has been shown to stimulate tall fescue growth under nitrogen limitation by altering regulation of phytohormones and nutrient homeostasis [88,89]. Other effects include alterations of plant gene expression, including upregulated expression of the HKT1 sodium transporter gene in shoots and downregulated expression in roots, which results in lower sodium accumulation throughout the plant [84,90].
In addition to PGPR isolated from soil around plant roots, endophytes (bacteria growing within plant tissues) have also been identified that stimulate plant growth [80,91,92]. Mechanisms by which endophytes enhance plant growth are thought to include enhanced nutrient acquisition and changes in host plant gene expression. For example, ACC (1-aminocyclopropane-1-carboxylase) deaminase is a bacterial enzyme found in many endophytes that stimulates nutrient acquisition and plant growth by reducing the amount of ACC converted to ethylene, a known inhibitor of plant growth, in response to salt, drought, and other environmental stresses [80,93,94]. Burkholderia phytofirmans is an endophyte that alters plant gene expression to enhance growth of six of the eight cultivars of switchgrass that were tested [91]. Inoculation with this strain was found to induce wide-spread changes in gene expression in the plant host, including altered expression of some transcription factors that are known to regulate the expression of plant stress factor genes [95]. Other bacterial endophytes (species of Sphingomonas, Pantoea, Bacillus, and Enterobacter) have been identified that enhance the salt tolerance of hybrid elephant grass [69], likely because of enhanced nutrient acquisition and/or gene expression changes [96].
3. Bacterial Strategies to Overcome Salinity Stress
Salt stress is one of the largest abiotic factors that can impact growth of an organism, including bacteria, which are also susceptible to osmotic stress. Halophilic bacteria have multiple mechanisms to counteract the osmotic stress of saline environments. There are two main types of adaptation mechanisms that halophiles use to prevent desiccation in the presence of salt: accumulation of water-soluble organic compounds in the cytoplasm and controlling the flux of inorganic ions. The main way that bacteria control the flux of inorganic ions is by exporting K+ ions to offset the influx of Na2+ ions. In addition, many halophiles utilize accumulation of water-soluble organic compounds (ectoine, hydroxyectoine, betaine, and choline) to offset the osmotic stress of highly saline environments. The accumulation of these compounds, or osmolytes, helps to draw water into the bacterial cell, preventing desiccation of the cell [97]. Another strategy that is employed by a wide variety of halophiles is controlling the flux of inorganic ions in the cell. If a cell has an influx of inorganic ions, this can lead to desiccation of the cell and eventual death of the organism. One method to prevent this influx of ions, typically sodium ions, is to actively pump intracellular potassium outside of the cell. This potassium typically comes in the form of KCl, and the export of KCl helps to offset the influx of NaCl from the saline environment [97].
It appears that in addition to the mechanisms used by PGPBs in general, there may be different mechanisms for PGPB stimulation of plant growth in saline conditions depending on the plant and bacterial species involved. The mechanism(s) by which halophilic bacteria stimulate plant growth may involve production of volatile compounds or other signals that stimulate expression of genes to enhance growth via increased photosynthesis [98] and other processes, including increased expression of plant membrane ion transport proteins. Other mechanisms that have been proposed include, but may not be limited to, the following ([67], Figure 1 and Table 1):
Table 1. List of selected studies reporting improved salt tolerance of field crops or phytoremediation by Kushneria and Halomonas species.
| Bacterial Species | Applications | Source of the Strain(s) | Experimental Conditions/Formulations/Outcome | Reference |
|---|---|---|---|---|
| Kushneria Species | ||||
| Kushneria sp. M3 | Bioremediation of saline soil polluted with petroleum hydrocarbons | The bacteria were isolated from the salt water and sediment of Red Sea, Jeddah, Saudi Arabia. | Bacterial consortium in continuous stirred tank reactor with petroleum refinery wastewater under saline condition (40 g/L NaCl concentration). Almost complete and 90% degradation of low- and high-molecular-weight PAHs, respectively, was observed. | [99] |
| Kushneria sp. YCWA18 | Increased germination under NaCl-alkali conditions as well as growth of Suaeda Salsa plants | The bacterium was isolated from the sediment of Daqiao saltern on the eastern coast of China. | The plants were inoculated with the bacteria and grown under NaCl-alkali regimes. A P solubilization of 616.98 mg/L was observed after 48 h. The germination was also improved. Bacteria did not show any effect under low salt-alkali conditions. | [100] |
| Kushneria marisflavi | Lowering salt-induced toxicity in barley, lettuce, and sunflower | Information about identification of bacterial strains not mentioned. | The seeds of the plants were inoculated with the bacterial culture, which was prepared using cells suspended in 2% NaCl solution, keeping an OD600 = 0.5 at 25 °C. The plants were grown in pots filled with sterilized sand and irrigated with NaCl-supplemented Hoagland’s solution. Plants were inoculated with bacterial culture twice. In the first treatment, seeds were soaked in bacterial culture for 45 min while the second inoculum was given after two weeks by adding 1 mL of bacterial culture to the medium. | [101] |
| Kushneria marisflavi | Improved alfalfa growth under salinity | Isolated from the soil and roots of Salicornia rubra, Sarcocornia utahensis, and Allenrolfea occidentalis. | Alfalfa seedlings grown at 1% NaCl concentration were inoculated with strains. The bacterial treatment of seedlings stimulated root growth in alfalfa up to 2.6-fold and a 21% increase in fresh weight compared to untreated controls. | [102] |
| Kushneria sp. | Elevated salt stress in rice | Different Bacteroidota and Actinobacteriota strains from Avicennia marina phyllosphere and rhizosphere were isolated. | The Kushneria strains inoculated were able to promote the growth of rice seedlings (root length, shoot length, and plant length) under 100 mM NaCl conditions by dissolving organic phosphorus and fixing nitrogen. The salt stress was applied by treating rice seeds with or without 100 mL NaCl solution while the inoculum was applied by adding 10 mL bacterial solution to the plates. | [103] |
| Kushneria | Enhanced salt tolerance in Chia | Different bacterial strains were isolated from the rhizosphere of Adesmia horrida (Fabaceae), Senecio punae (Asteraceae), and Pappostipa frigida (Poaceae). | Chia seeds were grown on half-strength MS medium supplemented with or without 50 and 100 mM NaCl. The bacterial strains were inoculated by adding a 20 µL bacterial culture to each plate, which were also prepared in half-strength MS medium, 0.2% sucrose, and 0.8% agar by culturing at 30 °C. | [104] |
| Kushneria BSSM27 | Alleviation of salt stress in durum wheat | The strains were isolated from the rhizosphere and roots of Halocnemum strobilaceum. | The cultures were prepared on YESA (Yeast Extract Sucrose Agar) medium with 2% sucrose and incubated at 30 °C. Plants were grown in pots, and inoculum (10 mL, OD600 = 0.6–0.8) was applied after coleoptile emergence. The salt stress was applied by irrigating plants with or without 100 mM and 200 mM NaCl solution every other day for 21 days. | [105] |
| Halomonas species | ||||
| Halomonas sp. Exo1 | Improved salt tolerance of rice plants in saline soils | The rhizobacteria was isolated from Avicennia marina rhizosphere of Indian Sundarbans. | The bacterium was applied either alone or in consortium with five other Halomonas strains. The treatment was applied twice: one before sowing to the seeds, and a second one at the time of transplantation of the seedlings into pots. Plants were grown in soil containing either 0.1% (w/w) NaCl or 0.2% (w/w) NaCl in the presence of arsenic. The treatment of bacterium alone did not yield any noticeable effect on germination of plants. A slight increase, however, occurred in the presence of arsenic. | [106] |
| Halomonas campaniensis 3H | Removal of nitrous oxide | The strain was isolated from a eutrophic saline lake sediment. | For denitrification experiments, the strain 3H was cultured in a minimal medium supplemented with 50 g/L NaCl, ammonia, nitrate, or nitrite as the sole nitrogen source, with constant shaking at 150 rpm and 30 °C. Cells from the logarithmic phase were diluted to OD600 = 0.02. Uninoculated medium was used as a control. An incubation of 96 h resulted in complete removal of ammonia. Further, addition of Cu+2 stimulated growth of 3H cells. | [107] |
| Halomonas sp. 3H | Salt tolerance in wheat | The strains were isolated from salt-tolerant rhizosphere. | A pot experiment was performed to assess the growth-promoting potential of the identified strains. Bacterial cultures were applied to wheat by soaking wheat seeds in bacterial cultures for 3–4 h. Plants without bacterial inoculation were used as control. Halomonas cell treatment significantly increased chlorophylls, carotenoids, and soluble sugars. Likewise, a beneficial effect on phenolic contents was also observed. | [108] |
| Halomonas sp. B01 | Removal of nitrogen (N) from high-salinity wastewaters | The strains were isolated from a saltern pool in Dalian, China. | The cells were cultured in a medium containing 30, 60, 90, and 120 g/L NaCl. For nitrogen removal, the strain was cultured in 5 mL media at 30 °C, and 1% of the cultures were inoculated in 300 mL flasks containing 30 mL of N removal medium; the SND was performed at 30 °C in a rotary shaker at 90 rpm. The strain was able to remove nitrogen in a time-dependent manner as well as a NaCl-dose-dependent manner. The removal efficiency of the strain was higher at high NaCl compared to lower concentrations, reaching as high as 90% in 96 h. | [109] |
| Halomonas sp. MAN5 | Enhanced root growth of Sesuvium portulacastrum under saline and heavy metal stress | The strains were isolated from soil samples collected from mangrove rhizosphere. | A pot experiment was conducted to study improvement in salt tolerance of S. portulacastrum. A 10 mL bacterial culture was used. Plants were irrigated with 2% NaCl saline water. The treatment was continued for 1 month and a number of plant growth parameters were recorded. The root growth and dry weights of the plants were increased 4-fold and 5-fold, respectively. | [110] |
| Halomonas sp. | Salt tolerance in purple basil | No information on how the strains were isolated was provided. | Bacterial cultures were prepared by adding 7% NaCl to LB broth. Basil plants were grown in pots filled with 0.5 L perlite. Bacterial solutions were applied after the emergence of cotyledons while salinity treatments were initiated after plants reached the 6–8 leaf stage. A one-fourth strength Hoagland’s solution containing 0, 50, 100, or 150 mM NaCl was used for irrigation. The stress was applied for three weeks. Different plant growth attributes were monitored. Halomonas treatment alone had no significant effect on plant growth. However, when used in combination with Azobacter sp., it significantly improved the growth parameters under even at 150 mM NaCl stress applied. | [111] |
| Halomonas sp. | Salt tolerance in maize | No information regarding the isolation of bacterial strains was provided. | The bacterial cultures for inoculation were prepared by culturing in LB medium containing 0.5 M NaCl at 37 °C overnight. To study the effect of salt tolerance in maize, plants were grown in pots and irrigated with 0, 50, 100, and 200 mM salt solution. Seeds were sterilized and inoculated with 10 mL bacterial suspension for 30 min. Uninoculated seeds were used as control. Seedlings were harvested after 15 days and different growth parameters were recorded. Bacterial treatments significantly improved growth of treated plants under NaCl stress. For example, an increase of up to 210% was noted in germination, an up to 40% increase in shoot length, and an up to 137% increase in root length compared to untreated controls. | [112] |
| Halomonas variabilis (HT1) | Improved growth of chickpea under salinity | No information provided about the isolation of strain. | The bacterial cells were applied by incubating chickpea seeds for 30 min. Plants were grown in pots containing 0, 50, 100, and 200 mM NaCl per gram of soil. Seedlings were harvested after 15 days, and several parameters including seedling length (cm), fresh weight (mg per seedling), and dry weight (mg per seedling) were noted. Bacterial inoculation stimulated germination by 152%. A 50% increase was noted in germination rate. The bacterial strain also positively increased both the fresh weight and dry weight by 153% and 1988% compared to control under salt stress. Soluble sugar contents were increased by 46% and protein contents were increased 107% under salt stress. A notable feature observed during the study was the soil aggregation to plant roots under salt stress. The Halomonas strain increased 666% in soil aggregation under NaCl stress. | [113] |
| Halomonas (MK873884) | Enhanced growth of alfalfa under salinity | Cells isolated from rhizosphere of halophytic species Salicornia rubra, Sarcocornia utahensis, and Allenrolfea occidentalis. | Alfalfa seedlings were first germinated in sterile water, and seedlings were shifted to magenta boxes containing autoclaved soil. Then, 100 mL half-strength Hoagland’s solution supplemented with 1% NaCl and 1 mL of bacterial suspension was added to each box. A second experiment was carried out in pots in a greenhouse. This time, 1 mL of PBS buffer with or without 1 mL of bacterial culture was added to each pot. Salt stress was initiated after 1 week of bacterial inoculation. For salt stress, plants were irrigated with or without 1% NaCl solution The stress was applied for one month. After one month, plants were harvested and different growth attributes noted. Significant changes in growth under salt stress were observed in plants treated with bacterial cells compared to untreated controls under similar conditions. For example, an up to 21% increase in fresh weight and up to a 2.6-fold increase in root length were observed in treated plants under stress conditions. | [102] |
| Halomonas BSSM328 | Alleviation of salt stress in durum wheat | The strains were isolated from the rhizosphere and roots of Halocnemum strobilaceum. | The cultures were prepared on YESA (Yeast Extract Sucrose Agar) medium with 2% sucrose and incubated at 30 °C. Plants were grown in pots, and inoculum (10 mL, OD600 = 0.6–0.8) was applied after coleoptile emergence. The salt stress was applied by irrigating plants with or without 100 mM and 200 mM NaCl solution after every other day for 21 days. | [105] |
| Halomonas venusta | Enhanced plant growth in sunflower | No information provided about the isolation and purification of strains. | The Helianthus annuus seeds were treated with or without bacterial cells cultured at 37 °C. Both the treated and untreated seed were grown in pots and harvested after one month. Plants treated with bacterial cells showed significant improvement in different plant growth attributes, such as shoot length (+136%), leaf number (+52%), protein content (+57%), and flower diameter (+31.4%). Likewise, a positive and statistically significant effect was observed on chlorophyll concentration. | [114] |
| Halomonas ventosae JPT10 | Promotes salt tolerance in foxtail millet, soybean, tomato, wheat, and maize | The cells were isolated from Suaeda salsa rhizosphere. | The cells were cultured in 15 mL LB medium containing 2 M NaCl at 28 °C for 24–48 h. The salt stress experiment was carried out in pots. The plants were irrigated with or without 100, 150, 200, and 250 mM NaCl solution. For bacterial inoculations, a 200 mL bacterial suspension was added to each pot. Bacterial treatment significantly improved plant growth under salt stress. The foxtail bacterial-treated plants accumulated fewer levels of OPDA, JA, MeJA, and ROS compared to untreated plants. Likewise, maize, wheat, soybean, and tomato bacterial-treated seedlings showed faster growth rates, and produced longer shoots and roots and higher fresh weights compared to untreated plants. | |
-
Some microbes produce biofilm/exopolysaccharides in the rhizosphere that trap water and nutrients and decrease plant uptake of sodium ions from the soil.
-
Some microbes inhibit growth of fungi and plant pathogens and/or select a certain microbial community in the rhizosphere.
-
Enhance plant access to nutrients.
-
Some microbes function as phytostimulators to produce ABA, IAA, and other plant hormones that stimulate shoot formation and plant growth by enhancing expression of specific plant genes.
-
Microbes can function as biofertilizers to produce nutrients or improve nitrogen fixation for the plant and/or enhance photosynthesis.
4. Salt Tolerant Bacterial Genus Kushneria
While much of the published work has focused on Bacillus species or other Gram-positive bacteria, some Gram-negative bacteria also have been shown to have PGPB potential. Two that have been shown to stimulate plant growth of salt-sensitive plants in salty soil are Kushneria and Halomonas (Table 1). Kushneria is a genus of bacteria in the Halomonadaceae family and comprises halophiles. The genus Kushneria was formed in 2009 when Halomonas marisflavi along with two other Halomonas strains were moved into this novel genus [116]. One of the most notable features of Kushneria is its ability to grow in high salt concentrations. Studies have shown that some species of Kushneria can grow in up to 25% NaCl concentration, which is higher than the salt concentration of seawater. This makes the genus an interesting subject of research for its potential use in bioremediation of saline soils, as well as in the production of salt-tolerant crops. Strains of Kushneria have been isolated from a variety of different salty environments, including a solar saltern, the leaves of black mangroves, sea water, salt mines, cured meats, and salt fermented foods [117,118,119,120,121,122,123]. Many species in this genus are adapted to hypersaline environments, and different strains have been isolated from the rhizosphere as well as the endosphere of halophytes [119,124]. These bacteria exhibit the ability to produce a variety of osmolytes, bioactive compounds (including betaine and ectoine that help protect from stress), and plant growth hormones [119,124]. Certain Kushneria species have been found to promote plant growth and act as biofertilizers or may function in phytoremediation, especially in saline agricultural soils [125]. A study by Parida and Das [126] revealed that Kushneria sp. NRCC 31,399 enhanced the growth of rice plants under saline conditions, indicating its potential use as a bioinoculant for salt-affected agricultural lands. Kushneria species are known for their ability to accumulate compatible solutes, which are small organic molecules that help the bacteria to cope with osmotic stress. These solutes have potential applications in various industries. In a study by Kulkarni et al. [127], Kushneria sp. GSB1 was found to produce the compatible solutes ectoine and hydroxyectoine in considerable amounts, highlighting the biotechnological potential of this genus in the production of these valuable compounds. Studies have shown that some species of Kushneria produce compounds that exhibit antimicrobial activity against various pathogenic bacteria and fungi. This suggests that Kushneria may have potential applications in the development of novel antibiotics and antifungal agents. Kushneria strains have been isolated from both the endosphere and the rhizosphere of plants and many exhibit the ability to produce a variety of plant hormones (Table 1). These examples demonstrate the diverse and promising biotechnological applications of Kushneria species. Continued research and exploration of this salt-tolerant bacterial genus may uncover even more practical uses in the future.
Kushneria and Halomonas bacteria have been isolated from a halophyte, Salicornia, in Tunisia, and some have been shown to have plant growth promotion activity [63]. A Kushneria marisflavi isolate in combination with Pseudomonas stutzeri was found to reduce salinity-stress-induced damage in lettuce and barley [101]. Despite the potential applications of Kushneria, much of its biology remains poorly understood. Further research is needed to fully elucidate the metabolic capabilities and genetic makeup of the genus. This would not only provide insights into the biology of Kushneria but also pave the way for the development of new biotechnological applications of the genus.
5. Salt-Tolerant Bacterial Genus Halomonas
Halomonas bacteria are able to grow in high-salt conditions and at high pH values. Halomonas can also resist contamination by other microbes, due to its ability to grow in highly saline and alkali conditions [91]. Halomonas spp. have been isolated from the endosphere of different plants, shrubs, and trees. They have been identified as Gram-negative, aerobic with yellow pigmentation, and are rod shaped [128]. Strains of Halomonas have been isolated from a variety of highly saline environments including salt marshes, the endosphere of halophytes, salt-cured meats, and fermented foods [125,128,129,130,131]. Bacteria from this genus have been found that produce a wide variety of diverse biochemicals and exopolysaccharides (EPSs) [129,130]. Table 1 includes other Halomonas strains that have been reported.
Some Halomonas species isolated from plants have been shown to have potential as PGPB [132,133] or in phytoremediation to improve soils [125] (Table 1). A collection of halotolerant microbes isolated from Salicornia ramiosissima [132] contains some isolates with PGPB activity. Two Halomonas and two Bacillus strains were identified from the rhizosphere of quinoa that have different activities as PGPB, and it was shown that these strains exhibit beneficial traits that are salt-regulated [133].
A consortium of five Halomonas strains was shown to improve salt tolerance of rice [106], suggesting that combinations of two or more strains may provide synergism. In wheat, inoculation with Halomonas sp. 3H led to an increase in chlorophylls, carotenoids, sugars, and phenolics [108]. Halomonas sp. MAN5 improved root growth of Sesuvium portulcastrum [110]. In separate studies, several other Halomonas strains have been shown to enhance salt tolerance in purple basil [111], maize [112], chickpea [131], and sunflower [114]. One Halomonas strain, H. ventosae JPT10, was tested with multiple plants and enhanced salt tolerance in foxtail millet, soybean, tomato, wheat, and maize [115], indicating that some strains may serve as PGPB for multiple crop species.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11122910
This entry is offline, you can click here to edit this entry!
