Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pathology
Calpain is defined as a member of the superfamily of cysteine proteases possessing the CysPC motif within the gene. Calpain-1 and -2, which are categorized as conventional isozymes, execute limited proteolysis in a calcium-dependent fashion. Accordingly, the calpain system participates in physiological and pathological phenomena, including cell migration, apoptosis, and synaptic plasticity.
- calpastatin
- calpain-10
- calpain-6
- calpain-1
- calpain-2
- conventional isozyme
1. Introduction
Obesity, diabetes, hypertension, and dyslipidemia are acknowledged to intersect via systemic metabolism, constituting potent common risk factors. This cluster of maladies is denoted as the metabolic syndrome, with its pernicious cycle of metabolic aberrations culminating in cardiovascular fatality [1,2]. In terms of diabetes, complications encompass chronic kidney diseases, significantly augmenting the propensity for coronary events [3,4]. Furthermore, dyslipidemia, as well as hyperglycemia and obesity, reportedly gives rise to intricacies involving steatohepatitis [5,6]. In contemporary times, this disease area has persistently broadened as a cardiometabolic disorder, prompting numerous epidemiological and molecular biomedical inquiries aimed at its prevention and amelioration. As a result of earlier studies, it was evidenced that statins have remarkable efficacy in rectifying dyslipidemia, with concomitant vasoprotective attributes [7,8]. Incretins and sodium glucose cotransporter 2 (sGLUT2) inhibitors are also ubiquitously employed in the realm of diabetes management, the former manifesting cardioprotective benefits as well as hepatoprotective effects [9,10], while the latter exhibit auspicious nephroprotective properties [11,12]. Hence, therapies endowed with pleiotropic and organoprotective attributes hold promise. Calpain is a stress-responsive intracellular protease, and its involvement in a variety of pathological conditions has been pointed out mainly through basic research since the early 2000s. This family of enzymes has been demonstrated to impart to atherosclerosis, as well as diabetes and hepatic affliction, thereby rendering it an exemplary molecular objective. A lot of chemical inhibitors targeting conventional calpain have been developed, and pharmaceutical companies have undertaken clinical investigations for diseases including Alzheimer’s disease and multiple sclerosis [13]. Given this context, calpain inhibitors have seemed clinically advantageous, and drug repositioning for cardiometabolic diseases may be considered promising. The therapeutic application of calpain inhibitors in cardiometabolic diseases is relatively behind, while recent basic research has elucidated the multifaceted actions of calpain in those metabolic disorders, indicating considerable anticipation for clinical applications.
2. Conventional Calpains and Calpastatin
Calpain designates a superfamily of cysteine proteases characterized by the presence of a CysPC motif within the gene (Figure 1). In mammals, there exist fifteen homologs of the catalytic subunits of calpains, each demonstrating diverse distributions and physiological functions in vivo [14,15,16,17,18]. Among these calpain isozymes, calpain-1 and calpain-2, categorized as conventional isozymes, have garnered global recognition since the 1970s [19,20]. Calpain-1 and calpain-2 form heterodimers by each binding with the common catalytic subunit, calpain-s1. They have undergone comprehensive examination using various methodologies across the fields of enzymology, physiology, and molecular pathology. Conventional calpains exhibit a notable sensitivity to calcium, a characteristic from which their nomenclature, “calpain”, is derived from “calcium” and “papain”. Their activity, however, is subject to regulation by extracellular stresses, including hypoxia, mechanical stresses, inflammatory cytokines, and growth factors, all mediated through the modulation of intracellular calcium levels [21]. In contrast, conventional calpains do not exhibit a strict recognition of a single amino acid sequence, and their substrate specificity remains relatively modest [22]. Conversely, the cleavage sites within each substrate exhibit precise delineation, frequently resulting in discrete fragments discernible as singular bands when subjected to Western blot analysis [23,24]. These substrate fragments may demonstrate divergent biological activities and stabilities compared to their full-length counterparts, thereby potentially influencing cellular functions. The distinct attributes of conventional calpains render them candidates for investigation in numerous disease etiologies. Nevertheless, unequivocally understanding their physiological functions is challenging due to the wide array of drivers and substrates.
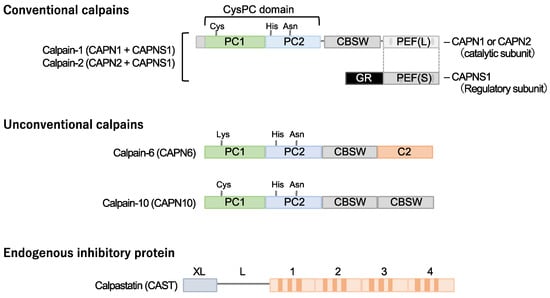
Figure 1. Cardiometabolic calpains. Calpains are a superfamily of cysteine proteases with a CysPc domain. Two types of conventional isozymes and two types of unconventional calpains are thought to be involved in cardiometabolic diseases. Conventional calpains are formed when the subtype-specific catalytic subunits calpain-1 and calpain-2 form heterodimers with the common regulatory subunit, calpain-s1, respectively. On the other hand, no quaternary structure has been identified for calpain-6 and calpain-10, which are unconventional isoforms. Calpastatin is known as a specific endogenous inhibitor, and its increased expression in cells may downregulate the activity of conventional isozyme and calpain-10. Notably, a cysteine residue in the active core of calpain-6 is substituted with lysine and is thought to lack protease activity. CBSW, calpain-type β-sandwich domain; CysPc, cysteine protease domain, calpain-type; GR, glycine-rich domain; PC, protease core; PEF, penta-EF-hand domain.
Calpastatin is a specific endogenous calpain inhibitor and is frequently colocalized with calpain proteases [23,25,26]. Structurally, calpastatin comprises a tandem repeat of inhibitory domains that directly interact with and inhibit calpain activity. It is established that the individual inhibitory domains exhibit significant inhibitory efficacy, adequate for the suppression of calpain activity [25]. Dysregulation of calpain-calpastatin balance has been associated with several pathological conditions, such as neurodegenerative diseases (e.g., Alzheimer’s disease and Huntington’s disease) [27,28,29,30], tumor angiogenesis [23], and retinopathy [23]. In these contexts, aberrant calpastatin expression or function can lead to increased calpain activity, contributing to disease progression.
3. Calpain-10
Several unconventional calpains are also known to contribute to cardiometabolic diseases. Calpain-10 is expressed in various tissues, including pancreatic β-cells [31]. It is comprised of a calpain-type β-sandwich domain and microtubule-interacting and transport domain, as well as CysPC protease domains, while a penta-EF-hand domain is lacking [20]. It was documented that calpain-10 is associated with several diseases, most notably type 2 diabetes mellitus. Its involvement in type 2 diabetes mellitus is linked to genetic variations in the CAPN10 gene, which encodes calpain-10 [32,33,34].
4. Calpain-6
Calpain-6 is categorized as an unconventional calpain devoid of proteolytic activity due to the substitution of the cysteine residue within the active site of the CysPC domain with lysine. Its molecular configuration also diverges from conventional calpains, lacking the penta-EF-hand domain responsible for binding to the regulatory subunit [19,20]. Moreover, it has not been documented in a heterodimeric form. The expression profile of calpain-6 exhibits extraordinary distinctiveness. Experimental investigations in murine models have revealed embryonic expression within skeletal and cardiac muscle tissue [35]. Nonetheless, postnatally, this expression is anticipated to wane, with persistent expression primarily confined to the placenta [36]. Notably, Capn6 (calpain-6 gene)-deficient mice exhibit heightened skeletal muscle capacity and expedited repair of damaged skeletal muscle [36], suggesting potential functional implications of calpain-6 in skeletal muscle development.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242316782
This entry is offline, you can click here to edit this entry!
