Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Advanced chronic liver disease (ACLD) represents a complex and multifactorial clinical entity characterized by liver dysfunction and associated complications.
- ACLD
- sarcopenia
- hepatic encephalopathy
1. Introduction
In recent years, the relationship between nutritional status and clinical outcomes in patients with advanced chronic liver disease (ACLD) has become increasingly clear with the increasing body of evidence published in the medical literature. ACLD refers to the advanced stage of liver disease characterized by structural changes of the liver parenchyma (histological evidence of liver fibrosis, regenerative nodules, and cirrhosis) and impaired liver function [1]. Malnutrition has been demonstrated to be associated with mortality, the development of complications, reduced survival, and reduced health-related quality of life (HRQoL) in individuals with ACLD [2,3,4].
Studies investigating the use of branched-chain amino acids (BCAAs), a group of essential amino acids, namely valine, leucine, and isoleucine, as a treatment for malnutrition in ACLD in adults provide contrasting findings. As such, the use of BCAA supplementation in clinical practice remains controversial, in particular with regards to the optimal dose, duration, and relative concentration of amino acids, as there was significant variability across the studies.
2. Branched-Chain Amino Acids (BCAAs) in Liver Disease
2.1. Low Fischer’s Ratio and BCAAs Metabolism in ACLD
A common characteristic observed in adults and children with ACLD is a decrease in serum BCAA concentration and an increase in aromatic amino acids (AAA), namely phenylalanine, tyrosine, and tryptophan [35], resulting in a low Fischer’s ratio (BCAA/AAA). The underlying cause of the low Fischer’s ratio in individuals with advanced liver disease has been attributed to increased oxidation and reduced endogenous disposal of BCAAs in skeletal muscle, along with alterations in AAA oxidation, indicating a higher BCAA requirement in chronic liver disease patients [36]. The metabolic changes in amino acid metabolism serve as a hallmark of liver disease and may become more pronounced with the progression of liver disease severity and the degree of malnutrition [37,38]. A low Fischer’s ratio has been found to be associated with the development of complications related to cirrhosis, such as hepatic encephalopathy [35].
BCAA derived from the diet largely bypasses first-pass hepatic catabolism, and their catabolic disposal primarily occurs in skeletal muscle, making them a preferred source of amino acids in patients with liver disease [39]. Numerous clinical trials have demonstrated the beneficial effects of BCAA supplementation in liver failure, including improvements in nutritional status, HE, and health-related quality of life (HRQoL) [40,41] (see Figure 1). Nonetheless, these results were not replicated in other studies [42], leaving a degree of uncertainty with regards to the potential therapeutic efficacy of the different outcomes of ACLD.
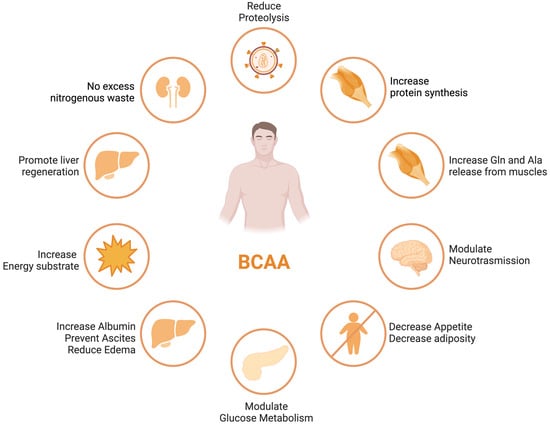
Figure 1. BCAA effects in different organs and tissues.
ACLD patients frequently exhibit decreased serum BCAA concentrations, which is associated with a poor prognosis [43,44]. The correlation between decreased levels of circulating BCAAs and the extent of liver disease severity, along with compromised muscle function, indicates the potential utility of BCAAs as a valuable prognostic indicator for gauging the progression of liver disease [45].
BCAAs have a stimulatory effect on protein synthesis and/or an inhibitory effect on proteolysis [43], and BCAA supplementation leads to an increase in the serum concentration of BCAAs in cirrhotic patients [46,47,48] (Figure 1). Among the BCAAs, leucine plays a particularly crucial role in enhancing muscle protein synthesis [49]. All three BCAAs share similar metabolic pathways [50] and have been shown to exhibit antagonism in their respective catabolism at different intake levels, emphasizing the importance of prescribing optimal concentrations of each BCAA to maximize their benefits [50] (Figure 2 and Figure 3A,B).
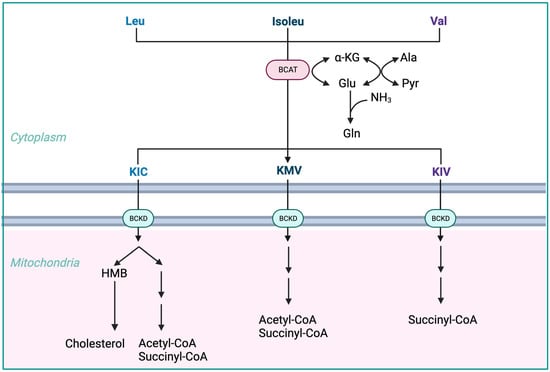
Figure 2. Advantages of BCAA-based nutritional formulas due to muscle dominance in BCAA catabolism. ALA—alanine; GLU—glutamate; GLN—glutamine; HMB—β-hydroxy-β-methylbutyrate; HMG-CoA—3-hydroxy-3-methylglutaryl-CoA; KIC—α-ketoisocaproate (ketoleucine); KIV—α-ketoisovalerate (ketovaline); KMV—α-keto-β-methylvalerate (ketoisoleucine); α-KG—α-ketoglutarate; BCAT—branched-chain-amino-acid aminotransferase; BCKD—branched-chain α-keto acid dehydrogenase.
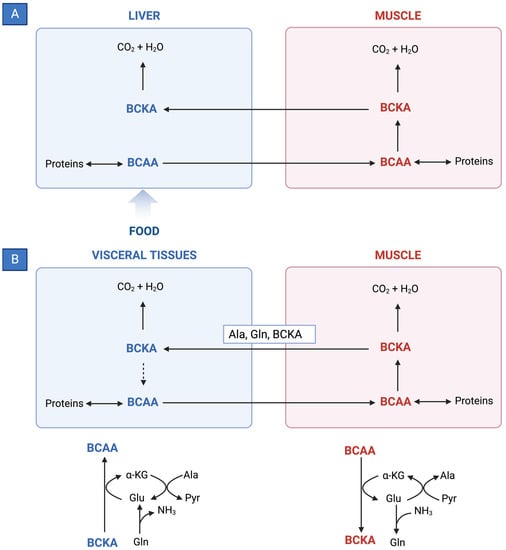
Figure 3. (A) Reversible BCAT reaction and amination process in tissue-specific BCAA metabolism. (B) Interorgan cycle: a mechanism to preserve BCAAs in physiological and pathological conditions. ALA—alanine; BCAAs—branched-chain amino acids; BCKAs—branched-chain keto acids; GLU—glutamate; GLN—glutamine; PYR—pyruvate; α-KG—α-ketoglutarate.
-
The initial stage of BCAA breakdown occurs outside the liver due to low BCAT activity, allowing BCAAs to quickly accumulate in the bloodstream, providing a unique advantage to BCAA-based nutritional formulas;
-
Skeletal muscle plays a vital role in BCAA breakdown, facilitated by branched-chain amino acid aminotransferase (BCAT). This leads to the production of branched-chain keto acids (BCKAs), glutamate, alanine, and glutamine, which are released into the bloodstream;
-
BCKD, an enzyme complex located in the inner mitochondrial membrane, irreversibly decarboxylates BCKAs into branched-chain acyl-CoA esters;
-
The activity of BCKD is regulated through phosphorylation and dephosphorylation processes mediated by specific kinases and phosphatases;
-
The liver exhibits the highest BCKD activity, while muscles, adipose tissue, and the brain have relatively lower activity levels;
-
Muscles, comprising a significant proportion of total body weight, are major contributors to overall BCAA utilization, alongside the liver;
-
Factors like cytokines, hormones, nutrients, and metabolites affect BCKD activity, with endotoxin or TNF-α administration inducing increased BCKD activity in muscles;
-
BCAAs follow diverse metabolic pathways post-BCKD reaction, with KIC being ketogenic, KIV being glucogenic, and KMV being both glycogenic and ketogenic;
-
The catabolism of KIC results in the synthesis of β-hydroxy-β-methylbutyrate (HMB) through the action of KIC dioxygenase.
2.2. Effects of BCAA Supplementation on Outcomes in ACLD
Recent nutritional guidelines from the European Association for the Study of Liver (EASL) [6] and the European Society of Parenteral and Enteral Nutrition (ESPEN) [52] recommend BCAA supplementation in subgroups of patients with liver cirrhosis, especially those with hepatic encephalothy (HE). The use of BCAAs is not exempt from mild adverse events. Potential adverse effects encompass esophageal reflux, enhanced insulin resistance, and disruptions in the sleep cycle.
2.2.1. Hepatic Encephalopathy
Hepatic encephalopathy (HE) refers to the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with advanced, acute, or chronic liver disease, secondary to hepatic dysfunction, portosystemic shunting, or both. It manifests as a wide spectrum of neuropsychiatric abnormalities, from subclinical changes (mild cognitive impairment) to marked disorientation, confusion, and coma.
The exact pathophysiological mechanism is not known, although it is increasingly recognized that hyperammonemia, systemic inflammation, intestinal dysbiosis, and portal-systemic shunting, leading to an increased flow of neurotoxins across the blood-brain barrier, act in a synergistic manner, leading to the development of HE [53].
This condition can significantly impact a patient’s quality of life and is associated with poor survival and a high risk of recurrence [2,5,54].
There is evidence that, in particular, oral BCAA-enriched formulations improve the manifestations of episodic HE (both overt or minimal) [7,55] while intravenous supplementation does not appear to have any beneficial effect [56].
A Cochrane systematic review including 16 randomized clinical trials compared BCAAs to placebo, diet, lactulose, or neomycin in people with cirrhosis. The results showed that BCAAs had a beneficial effect on manifestations of HE, including overt hepatic encephalopathy (RR = 0.73, 95% CI 0.61–0.88) but not minimal hepatic encephalopathy. There were no beneficial or detrimental effects of BCAAs on mortality, quality of life, or nutritional outcomes [57].
A recent high-quality randomized clinical trial showed BCAAs did not prevent recurrence in patients with a previous episode of overt HE [35].
2.2.2. Overall Survival
A recent meta-analysis of six studies was conducted to assess the effect of BCAA supplementation on overall survival in cirrhotic patients [13]. The studies included a total of 1253 patients who were followed for at least 6 months. The results showed that BCAA supplementation was not statistically significantly associated with an improvement in overall survival (RR = 0.58, 95% CI 0.34–1.00). However, one study found a statistically significant beneficial effect of BCAA supplementation on overall survival during hospital admission (mortality 12% vs. 47% in the supplemented and non-supplemented groups, respectively) [58]. The other studies did not find an effect of BCAA supplementation on overall survival [59,60,61].
As previously mentioned, no benefit to survival was found in Gluud’s Cochrane systematic review [57].
2.2.3. Event-Free Survival
In the same meta-analysis of six studies, van Dijk et al. reviewed the effect of BCAA supplementation on event-free survival in ACLD patients [13]. The studies included a total of 1035 patients who were followed for at least 6 months (follow-up period from 1 to 3 years). The dosage of BCAA supplementation was approximately 12 g per day, and the tracked cirrhosis-related events included death, liver decompensation, and hepatocellular carcinoma. The results showed that BCAA supplementation was associated with a statistically significant improvement in event-free survival (RR = 0.61, 95% CI 0.42–0.88). As such, patients who received BCAA supplementation were 61% less likely to experience an event (such as hospitalization, liver decompensation, or death) than patients who did not receive BCAA supplementation. Four of the studies included in the meta-analysis were randomized controlled trials [4,62,63,64], one was a prospective study [65], and one study had a retrospective design [65]. When only the randomized controlled trials were included in the meta-analysis, a significant effect on event-free survival was still present (RR = 0.70, 95% CI 0.54–0.91).
2.2.4. Nutritional Status
The same systematic review gathered eight available studies on BCAA supplementation and nutritional parameter outcomes [13]. The results of these studies are contradictory, as some show beneficial effects of BCAA supplementation and others show no effect or even harmful effects. A recent placebo-controlled trial on BCAAs in cirrhotic patients with sarcopenia found that BCAA supplementation had a beneficial effect on skeletal muscle index measured with a CT scan, but this study was small and did not correct for other factors that could have influenced the results [61].
2.2.5. Quality of Life
Three out of the five studies reported in van Dijk’s systematic review found that BCAA supplementation improved one or more subscales of the SF-36, a health-related quality of life questionnaire [4,63,66]. No effect was reported in the remaining two studies [3,67]. The studies included a total of 875 patients, with 442 receiving BCAA supplementation and 433 receiving a placebo [13].
2.2.6. Liver Disease Severity and Hepatocellular Carcinoma
Limited or conflicting evidence is available for the effectiveness of BCAA supplementation in most subgroups of chronic liver disease following specific therapeutic interventions for hepatocellular carcinoma (HCC) [68].
Van Dijk and colleagues conducted a systematic evaluation to assess the potential effects of BCAA supplementation in patients with chronic liver disease and coexisting HCC [68]. They examined various clinical circumstances, including general chronic liver disease as well as specific treatment situations such as hepatic resection, radiological intervention, systemic therapy for HCC, liver transplantation, large volume paracentesis for ascites, and endoscopic therapy of esophageal varices. The studies included a total of 1252 patients, with 581 patients receiving BCAA supplementation and 671 patients receiving placebo. The follow-up period of the studies varied between 1 and 3 years. No significant difference was found in the occurrence rates of HCC between the BCAAs and placebo groups (RR = 0.82, 95% CI 0.60–1.12). The exception was systemic therapy (sorafenib), where the two available retrospective studies reported better outcomes in patients receiving long-term BCAA supplementation compared to the control group [62,63].
The analysis focused on the effects of BCAA supplementation on various parameters reflecting liver function (Child–Pugh and MELD scores, serum albumin, clotting, and bilirubin concentrations) and revealed contradictory results with no overall significant effect.
Similarly, the effects of BCAA supplementation on sarcopenia showed inconsistent findings across different assessment methods (bioelectrical impedance analysis [7], mid-arm muscle circumference [9], and CT scan to measure skeletal muscle index [50]). No beneficial effects of BCAA supplementation on sarcopenia were reported in patients who underwent specific therapeutic interventions for HCC, such as resection, trans-arterial chemoembolization (TACE), or radiotherapy [68].
This entry is adapted from the peer-reviewed paper 10.3390/nu15194190
This entry is offline, you can click here to edit this entry!
