Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Environmental
Extracellular electron transfer (EET) is a biological mechanism that plays a crucial role in various bioelectrochemical systems (BESs) and has substantial implications for renewable energy production. By utilizing the metabolic capacities of exoelectrogens, BESs offer a viable and environmentally friendly approach to electricity generation and chemical production; however, the diminished effectiveness of EET remains a hindrance to their optimal application in practical contexts.
- extracellular electron transfer
- bioelectrochemical systems (BESs)
- direct electron transfer (DET)
- indirect electron transfer (IET)
- microorganisms
- temperature
- pH
- electron acceptors
1. Introduction
Microorganisms have devised specific processes to transmit electrons to minerals that are located near the surface of the cell, and this process is termed extracellular electron transfer (EET) [1]. The microbial EET process serves as a source of energy for the growth and maintenance of microorganisms. These microorganisms capable of conducting the EET process are referred to as electroactive microorganisms (EAMs) [2]. EAMs are now used for many applications in many environmental biotechnologies such as energy recovery and environmental remediation. For bioelectrochemical systems like microbial electrolysis cells (MECs) and microbial fuel cells (MFCs), the power production, fuel generation rates, and overall energy recovery from organic matter are all improved by increasing EET efficiency [3]. In addition to the generation of bioelectricity, the biotransformation of valuable chemicals, and the bioremediation of environmentally hazardous pollutants, EAMs are now useful for biosensing and even biocomputing [4]. EET enhancement is important in studying and harnessing microbe–metal interactions [5,6,7,8,9], but the complexity of electron transfer mechanisms, the wide range of microbes in question, the influence of environmental factors, and the scaling-up of EET processes are among the key challenges that researchers and engineers face in this field [9,10]. Overcoming these challenges will pave the way for the development of innovative applications for harnessing the potential of EET.
There are now several techniques used in environmental biotechnology to improve EET. To improve the EET capabilities of EAMs like Shewanella or Geobacter species, genetic engineering approaches can be used. This includes adding exogenous electron transfer mediators or overexpressing crucial EET genes or pathways [3]. Biofilm growth can also improve EET between microorganisms and electrodes. Optimizing growth conditions, surface modification, and biofilm engineering approaches are methods to improve the formation of biofilm [11,12]. Another method of improving EET is to optimize the material of the electrode. EET can be enhanced by modifying the composition and surface features of the electrodes [13,14,15]. This includes enhancing the contact between microbes and electrodes by employing conductive materials, such as carbon nanotubes or conductive polymers [2]. Redox mediators like riboflavin, neutral red, or anthraquinone derivatives can improve EET by promoting electron transport between microorganisms and solid surfaces or electron acceptors [16,17].
Electrochemical techniques are essential in studying the behavior of microorganisms for various purposes. One popular electrochemical method for examining the redox behavior of electroactive species in a system is cyclic voltammetry (CV). CV is used in bioelectrochemical systems to describe the electrochemical activity of redox-active mediators and microorganisms involved in EET. CV offers insights into the kinetics and thermodynamics of EET processes by assisting in determining the redox potentials of electron-donor and electron-acceptor compounds. Chronocoulometry (CC) is an additional electrochemical method utilized to quantify the overall electric charge that traversed an electrochemical cell within a designated time interval. This method can be performed by imparting a constant potential to the working electrode and measuring the resulting current as a function of time. The term “chronocoulometry” is derived from the words “coulometry”, which quantifies the amount of electricity (charge) exchanged within a system, and “chrono”, which denotes time. Pyocyanin (PYO) is an identified redox mediator molecule produced by Pseudomonas aeruginosa. The electrochemical technique utilizing CV and CC was employed in a recent study [18], to facilitate the analysis of the adsorption process of PYO onto carbon-based electrodes using glassy carbon. By utilizing these electrochemical methods in conjunction with FT-IR analysis, the authors deduced that P. aeruginosa NEJ07R establishes electronic communication with the electrode via PYO adsorption on the carbon surface. The research endeavors to enhance the engagement between redox mediators, microorganisms, and electrodes. This, in turn, may contribute to the advancement of microbial electrochemical technologies that address ecological challenges including, but not limited to, waste valorization, wastewater treatment, and renewable energy generation.
Gaining a comprehensive understanding of the electrochemical characteristics exhibited by bio-anodes is crucial to enhancing the overall efficiency and effectiveness of bioelectrochemical systems. Electrochemical impedance spectroscopy (EIS) is frequently employed for the comprehensive investigation of these characteristics. In a study conducted by Heijne et al. [19], the authors aimed to measure the properties of bio-anodes, specifically biofilm capacitance, charge transfer, biofilm resistance, and diffusion resistance. To ensure accurate measurements without the influence of electrode capacitance, the researchers utilized Fluorinated Tin Oxide (FTO) as the electrode material for cultivating the electroactive biofilm. FTO has favorable characteristics as an electrode material in the electrochemical investigation of bio-anodes. This is primarily due to its exceptional stability and significantly lower capacitance, which typically ranges in the tens of microfarads per square centimeter (μF cm−2), in contrast to conventional carbon electrodes, such as graphite plates, that possess a capacitance of approximately 1 millifarad per square centimeter (~1 mF cm−2). The researchers conducted a study to observe the formation of an electroactive biofilm on FTO (by the utilization of EIS and polarization experiments). The findings of the study demonstrate that the capacitance of the biofilm exhibited a significant rise from an initial value of 2 μF cm−2 to a final value of 450 μF cm−2 as the biofilm developed. This observation suggests a correlation between the capacitance and both the current and the total charge generated. The findings of the study additionally indicate that biofilm capacitance can serve as an indicator of the quantity of active biomass present in bioelectrochemical systems.
Scanning electrochemical microscopy (SECM) is an additional method that enables the visualization of electrochemical phenomena at the microscale. In the field of bioelectrochemical systems, this technique can be employed to see and analyze the spatial distribution of electroactive species as well as the level of microbial activity on electrode surfaces. SECM offers valuable insights into the electrochemical activity of biofilms, enabling a deeper understanding of the variability in electron transport mechanisms across microbial communities. The technique has also demonstrated its utility in various applications, including the evaluation of membrane permeability, neurotransmitter levels, and intracellular parameters [20].
Furthermore, the utilization of potentiostatic and galvanostatic operation is an electrochemical technique wherein the potential or current, accordingly, is regulated within an electrochemical system. Within the field of bioelectrochemical systems, potentiostatic and galvanostatic techniques are employed to manipulate the electrochemical conditions, modulate the rate of electron transfer, and examine the reaction of microorganisms to varying applied potentials or currents [21,22]. These electrochemical techniques play an important role in the characterization, optimization, and understanding of the intricate mechanisms associated with extracellular electron transfer in bioelectrochemical systems. Their contributions are crucial in the advancement of microbial electrochemistry and the pragmatic implementation of these systems in the fields of energy generation, wastewater treatment, and environmental monitoring.
2. Direct Electron Transfer (DET)
Microorganisms can carry out EET in different ways as depicted in Figure 1. For DET, electrons can move from the bacteria to electrodes independent of external mediators. This method necessitates the proximity of microorganisms to the outer electron recipient and entails the transfer of electrons via outer membrane proteins known as c-type cytochromes [23,24]. The limited distance of electron transfer in DET is known to be a contributing factor to the improved EET process. The transfer of electrons through the cytochrome pathway has been extensively studied in Geobacter sulfurreducens. This has been achieved through the use of mutant strains, wherein the gene responsible for encoding cytochrome c proteins has been either overexpressed or deleted [25].
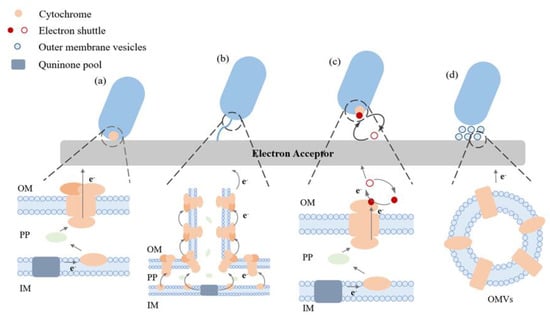
Figure 1. Elucidating the extracellular electron transport pathways that are commonly observed in electroactive microorganisms. (a) DET facilitated by cytochrome proteins, (b) the process of DET facilitated through nanowires, (c) the mode of IET facilitated by electron shuttles, (d) the method of indirect electron transfer facilitated through exterior vesicles of the membrane (OMVs). Outer membrane (OM), interior membrane (IM), and periplasm (PP).
The DET process is not exhibited by all microorganisms; hence, there has been a focus on investigating an alternative mechanism that is comparable but less comprehended, referred to as microbial nanowires. This mechanism has been occasionally referred to as a variant of DET. Nanowires are cellular membrane protrusions that bear a resemblance to pili. Due to their relatively elevated conductivity, it is postulated that they facilitate the conduction of electrons. Consequently, this confers an advantage to microorganisms by enabling them to inhabit a location that is distanced from the metal oxide or electrode. Additionally, this could facilitate inter-species electron transfer within a biofilm [23].
3. Indirect Electron Transfer (IET)
In IET, microorganisms can generate and secrete diminutive molecules that can undergo redox cycling, thereby acting as an ES (Figure 1). They function as the final electron acceptor and, upon reduction, are capable of transferring electrons to iron oxides, subsequently undergoing reoxidation. This type of shuttle offers a means for an indirect reduction process. The repetitive cycling of a solitary shuttle molecule can significantly influence the turnover rate of the terminal oxidant, such as iron, within a specific situation. Compounds such as humic compounds, quinones, phenazines, and molecules containing thiols have the potential to fulfil this function [26,27,28,29]. Electron shuttles can be classified into two distinct categories, specifically exogenous and endogenous, depending on their presence in the environment or the inherent ability of the cell to produce them. Thionine is the predominant exogenous mediator utilized, while other compounds such as quinine, phenoxazine, phenazine, and phenothiazine have also been employed in this capacity. On the other hand, endogenous mediators are released from cells in a reduced state and undergo oxidation to enable electron transfer at the electrode. The mediators under consideration in this category consist of flavin compounds produced by S. oneidensis MR-1 and 2-amino-3-carboxy-1,4-naphthoquinone, which is excreted by S. putrefaciens [24].
4. Factors Affecting EET Efficiency
EET is subject to the influence of multiple factors, encompassing temperature, pH levels, the existence of electron acceptors and donors, redox potential, as well as the properties of the electrode. Temperature affects the rate of chemical reactions and the availability of electron transfer [30]. High temperatures can enhance EET efficiency by increasing the rate of enzymatic reactions involved in electron transfer. The pH level is an essential determinant that affects both the activity of enzymes involved in EET and the proton gradient across cellular membranes. Enzymes involved in extracellular electron transfer often exhibit pH-dependent activity, and an increase in pH can disrupt the proton gradient and impede EET efficiency [31]. Electron acceptors and donors are also critical factors in EET efficiency. Oxygen is a highly favorable electron recipient because of its strong reduction potential and abundance in many natural environments [32]. In environments with a limited availability of electron acceptors, microorganisms may employ alternative strategies for EET. Organic substances like sugars, fatty acids, alcohols, and hydrogen gas are typical electron donors [33]. Redox potential is another crucial factor in EET processes. Microorganisms can utilize various electron acceptors with different redox potentials, such as solid metal oxides and metals like gold and platinum. The redox potential of these metal oxides influences their ability to accept electrons from microbial donors [34]. Electrode properties also play a significant role in EET efficiency. Conductive materials like graphite, carbon nanotubes, and metals like gold and platinum are often employed as electrodes because they possess strong electrical conductivity [35,36]. Biochar, a representative carbon-based material, has also been shown to serve as an ES to mediate the DIET process of Methanosarcina and Geobacter [37]. Thus, temperature, pH level, the presence of electron acceptors and donors, redox potential, and the characteristics of the electrodes are a few factors that generally affect the efficiency of EET.
This entry is adapted from the peer-reviewed paper 10.3390/app132312760
This entry is offline, you can click here to edit this entry!
