Antibiotic resistance is an increasing global problem for public health, and focusing on biofilms has provided further insights into resistance evolution in bacteria.poorly treated bacterial infections can lead to failures of different organ systems, particularly in fragile patients, and excessive immune-mediated inflammatory responses during sepsis may occur more frequently. Is it possible that nature can help control AMR diffusion? An analysis of resistance mechanisms is summarized, and an excursus of the different approaches to challenging resistance spread based on natural processes is presented as “lessons from Nature”. On the “host side”, immunotherapy strategies for bacterial infections have a long history before antibiotics, but continuous new inputs through biotechnology advances are enlarging their applications, efficacy, and safety. Antimicrobial peptides and monoclonal antibodies are considered for controlling antibiotic resistance. Understanding the biology of natural predators is providing new, effective, and safe ways to combat resistant bacteria. As natural enemies, bacteriophages were used to treat severe infections before the discovery of antibiotics, marginalized during the antibiotic era, and revitalized upon the diffusion of multi-resistance. Finally, sociopolitical aspects such as education, global action, and climate change control are also considered as important tools for tackling antibiotic resistance from the One Health perspective.
- antibiotic resistance
- biofilm and quorum sensing
- antimicrobial peptides
1. Introduction
2. Antibiotics’ Primary Targets and Resistance Mechanisms
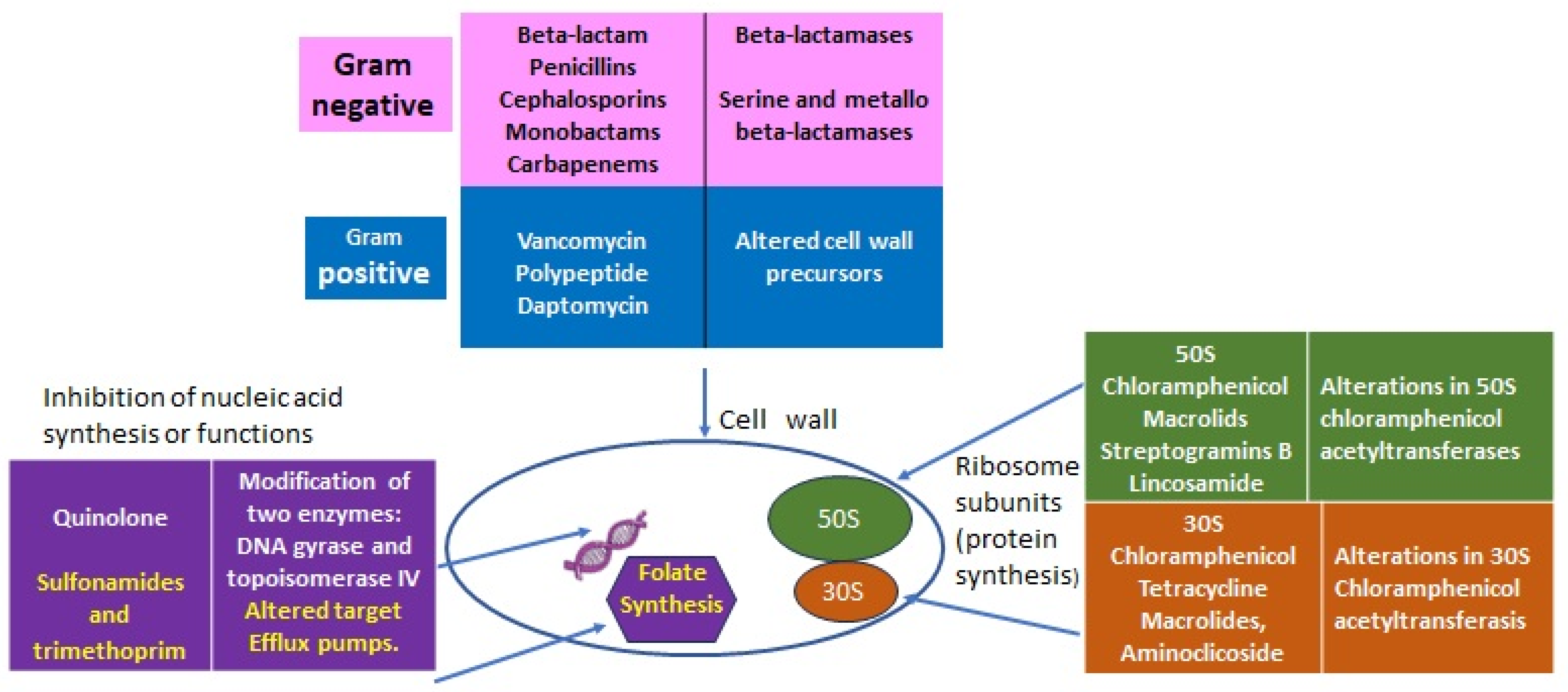
2.1. Lessons from Nature: From Intrinsic to Acquired Resistance
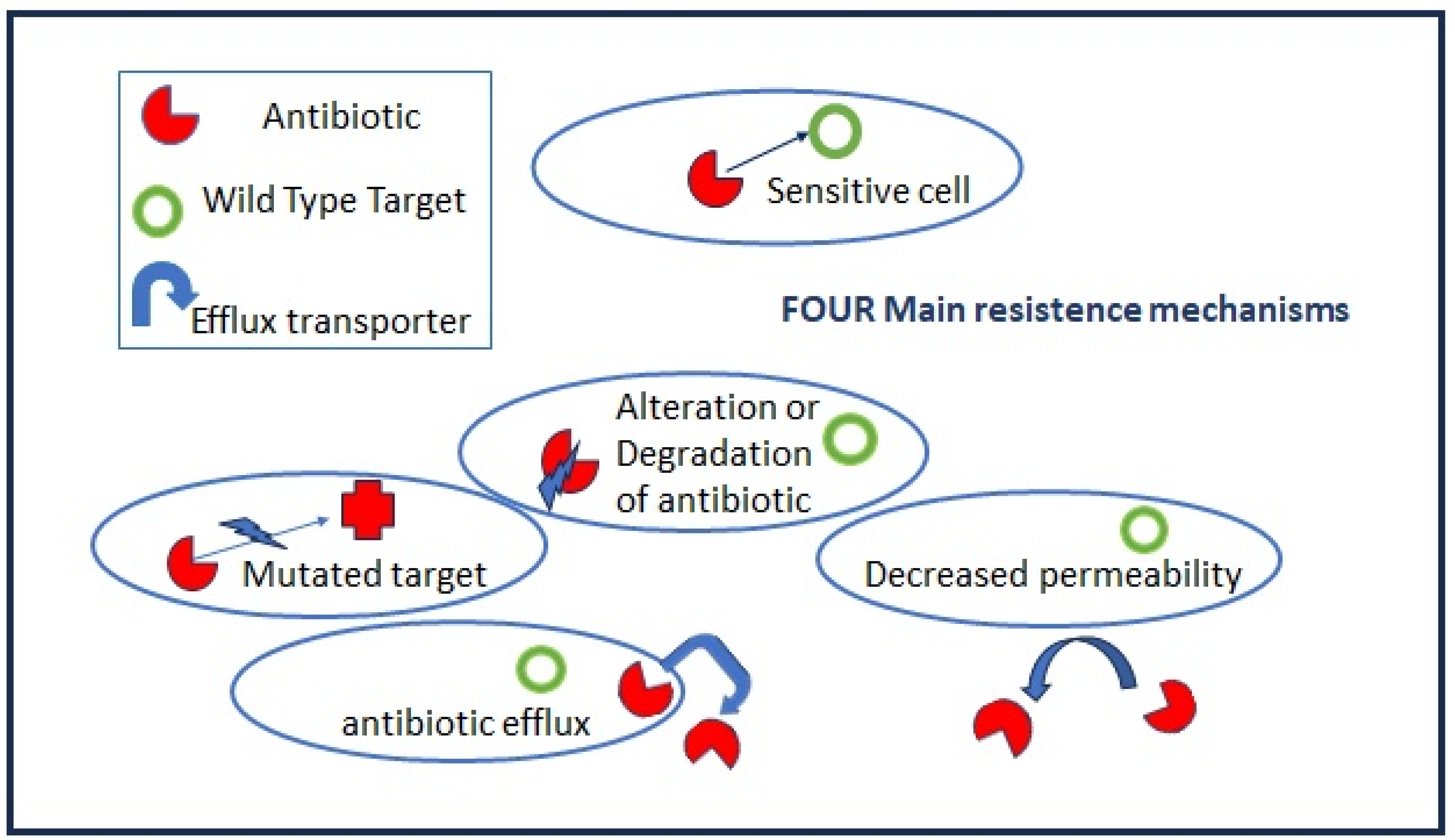
2.1.1. Intrinsic Resistance
| Intrinsic Resistance (IR) | Determinants | |
|---|---|---|
| All Gram-negative diderm bacteria | Glycopeptides, lipopeptides, and antibiotic targeting the bacteria peptidoglycan wall | EPS (extra-polymeric substance) avoids the permeability of antibiotics. |
| P. aeruginosa | Sulfonamides, ampicillin, 1st- and 2nd-generation cephalosporins, chloramphenicol, and tetracycline | Constitutive expression of Amp C beta-lactamase and efflux pumps. Low permeability of the outer membrane [6]. |
| Enterococcus spp. | Aminoglycosides, cephalosporins, and lincosamides | Low cell wall permeability, aminoglycoside-modifying enzyme (AME), ribosome-modifying methyltransferase, altered cell wall, and ABC-efflux pump [5]. |
| L. monocytogenes | Cephalosporins | Penicillin-binding proteins, multidrug resistance transporters, cell envelope proteins, etc. [3]. |
| E. coli | Macrolides | Macrolides modifying genes such as mphA; efflux pump [7]. |
| K. pneumonia | Ampicillin | SHV beta-lactamase, the fosfomycin resistance gene fosA, and the nalidixic acid efflux pump OqxAB [8]. |
| A. baumanii | Cephalsporins, ampicillin, glycopeptides, and carbapenems | Class C (AmpC) and Class D beta-lactamases located in chromosome [9]. |
2.1.2. Acquired Resistance
- (a)
-
Mutations in target genes located at chromosomal or extrachromosomal elements which are vertically transmitted in the same bacteria species. The mutation rate increases when bacteria are actively multiplying as, for example, during the acute phase of host infection.
- (b)
-
Horizontal gene transfers occur through mobile elements that can be transmitted both intraspecies and among different bacteria genera, i.e., the vancomycin-resistant gene (vanA) from Enterococcus to S. aureus. Plasmids, prophages, pathogenicity islands, restriction and modification systems, transposons, and insertion sequences are able to move within the host genome as well as jump across genomes. Mobile elements can change their insertion location and copy number and produce frequent gene gain and loss, modifying and co-evolving with chromosomal genomes. The genetic modifications induced by mobile elements can deeply affect bacterial fitness, contributing to their adaptation to new environments and, ultimately, producing evolutionarily distinct species over time. Once the acquisition of resistance determinants is established in few strains, antibiotic misuse and pressure drive the positive selection of resistant over sensitive strains.
2.1.3. Acquired Resistance through Bacterial Cooperation
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12121694
References
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient antibiotic use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66, vi3–vi12.
- Youle, M.; Rohwer, F.; Stacy, A.; Whiteley, M.; Steel, B.C.; Delalez, N.J.; Nord, A.L.; Berry, R.M.; Armitage, J.P.; Kamoun, S.; et al. The Microbial Olympics. Nat. Rev. Microbiol. 2012, 10, 583–588.
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; pp. 1–7.
- Krawczyk-Balska, A.; Markiewicz, Z. The intrinsic cephalosporin resistome of Listeria monocytogenes in the context of stress response, gene regulation, pathogenesis and therapeutics. J. Appl. Microbiol. 2016, 120, 251–265.
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–569.
- Strateva, T.; Yordanov, D. Pseudomonas aeruginosa—A phenomenon of bacterial resistance. J. Med. Microbiol. 2009, 58 Pt 9, 1133–1148.
- Nguyen, M.C.P.; Woerther, P.L.; Bouvet, M.; Andremont, A.; Leclercq, R.; Canu, A. Escherichia coli as reservoir for macrolide resistance genes. Emerg. Infect. Dis. 2009, 15, 1648–1650.
- Bernardini, A.; Cuesta, T.; Tomás, A.; Bengoechea, J.A.; Martínez, J.L.; Sánchez, M.B. The intrinsic resistome of Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2019, 53, 29–33.
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373.
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641.
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890.
- Yurtsev, E.A.; Conwill, A.; Gore, J. Oscillatory dynamics in a bacterial cross-protection mutualism. Proc. Natl. Acad. Sci. USA 2016, 113, 6236–6241.
- Dawan, J.; Ahn, J. Assessment of cooperative antibiotic resistance of Salmonella Typhimurium within heterogeneous population. Microb. Pathog. 2021, 157, 104973.
