G protein-coupled receptors (GPCRs) are transmembrane proteins that mediate the intracellular pathway of signals not only through heterotrimeric GTP-binding proteins (G proteins) but also through their associations with a variety of additional partner proteins. Prokineticin receptors 1 (PKR1) and 2 (PKR2) are new members of the GPCRs whose ligands are the novel chemokines prokineticin 1 (PK1) and prokineticin 2 (PK2). The multiplicity of G proteins coupled to PKRs, the ability of PKR2 to heterodimerize, the interaction of PKR2 with accessory proteins, and the existence of alternative splice isoforms of PKR2/PK2 explain the complexity of the system in the signal transduction pathway and, consequently, in the modulation of various physiological and pathological functions. Knowledge of these mechanisms provides the basis for the development of targeted drugs with therapeutic efficacy in PK-dependent diseases.
- G protein-coupled receptors
- accessory proteins
- prokineticins
- prokineticin receptors
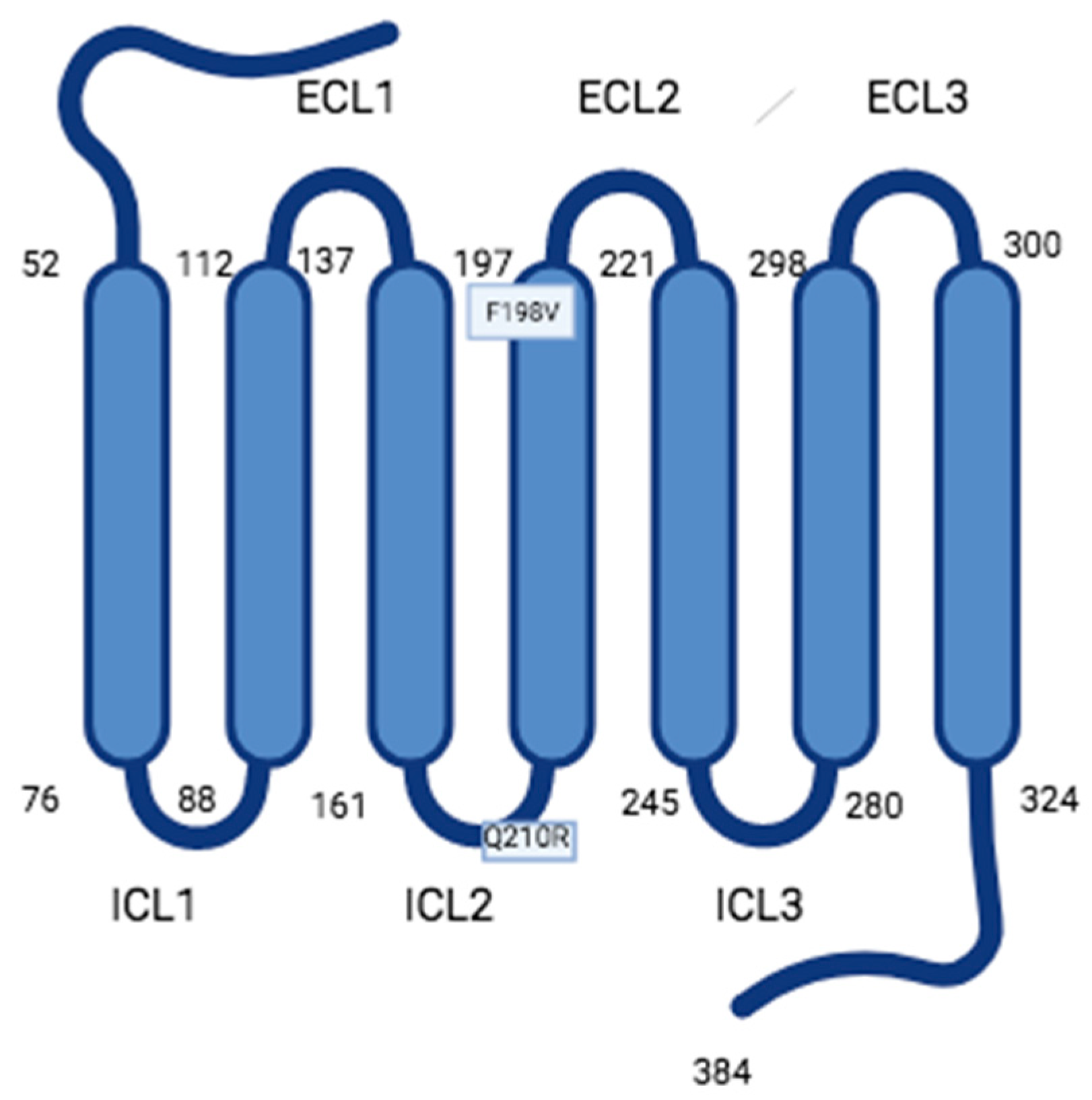
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia3040107
References
- Rosenbaum, D.M.F.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363.
- Soga, T.; Matsumoto, S.; Oda, T.; Saito, T.; Hiyama, H.; Takasaki, J.; Kamohara, M.; Ohishi, T.; Matsushime, H.; Furuichi, K. Molecular cloning and characterization of prokineticin receptors. Biochim. Biophys. Acta 2002, 1579, 173–179.
- Lin, D.C.; Bullock, C.M.; Ehlert, F.J.; Chen, J.-L.; Tian, H.; Zhou, Q.Y. Identification and Molecular Characterization of Two Closely Related G Protein-coupled Receptors Activated by Prokineticins/Endocrine Gland Vascular Endothelial Growth Factor. J. Biol. Chem. 2002, 277, 19276–19280.
- Masuda, Y.; Takatsu, Y.; Terao, Y.; Kumano, S.; Ishibashi, Y.; Suenaga, M.; Abe, M.; Fukusumi, S.; Watanabe, T.; Shintani, Y.; et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem. Biophys. Res. Commun. 2002, 293, 396–402.
- Negri, L.; Ferrara, N. The Prokineticins: Neuromodulators and Mediators of Inflammation and Myeloid Cell-Dependent. Angiogenesis. Physiol. Rev. 2018, 98, 1055–1082.
- Lattanzi, R.; Maftei, D.; Fullone, M.R.; Miele, R. Identification and characterization of Prokineticin receptor 2 splicing TM4-7 variant and its modulation in an animal model of Alzheimer's disease. Neuropeptides 2019, 73, 49–56.
- Li, M.; Bullock, C.M.; Knauer, D.J.; Ehlert, F.J.; Zhou, Q.Y. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol. 2001, 59, 692–698.
- Mollay, C.; Wechselberger, C.; Mignogna, G.; Negri, L.; Melchiorri, P.; Barra, D.; Kreil, G. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur. J. Pharmacol. 1999, 374, 189–196.
- Schweitz, H.; Pacaud, P.; Diochot, S.; Moinier, D.; Lazdunski, M. MIT (1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett. 1999, 461, 183–188.
- Ferrara, N.; LeCouter, J.; Lin, R.; Peale, F. EG-VEGF and Bv8: A novel family of tissue-restricted angiogenic factors. Biochim. Biophys. Acta 2004, 1654, 69–78.
- LeCouter, J.; Kowalski, J.; Foster, J.; Hass, P.; Zhang, Z.; Dillard-Telm, L.; Frantz, G.; Rangell, L.; DeGuzman, L.; Keller, G.A.; et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001, 412, 877–884.
- Lattanzi, R.; Miele, R. Prokineticin-Receptor Network: Mechanisms of Regulation. Life 2022, 12, 172.
- Jilek, A.; Engel, E.; Beier, D.; Lepperdinger, G. Murine Bv8 gene maps near a synteny breakpoint of mouse chromosome 6 and human 3p21. Gene 2000, 256, 189–195.
- Chen, J.; Kuei, C.; Sutton, S.; Wilson, S.; Yu, J.; Kamme, F.; Mazur, C.; Lovenberg, T.; Liu, C. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol. Pharmacol. 2005, 67, 2070–2076.
- Lattanzi, R.; Maftei, D.; Negri, L.; Fusco, I.; Miele, R. PK2β ligand, a splice variant of prokineticin 2, is able to modulate and drive signaling through PKR1 receptor. Neuropeptides 2018, 71, 32–42.
- Lattanzi, R.; Maftei, D.; Vincenzi, M.; Fullone, M.R.; Miele, R. Identification and Characterization of a New Splicing Variant of Prokineticin 2. Life 2022, 12, 48.
- Kaser, A.; Winklmayr, M.; Lepperdinger, G.; Kreil, G. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep. 2003, 4, 469–473.
- Monnier, J.; Samson, M. Cytokine properties of prokineticins. FEBS J. 2008, 275, 4014–4021.
- Lin, R.; LeCouter, J.; Kowalski, J.; Ferrara, N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J. Biol. Chem. 2002, 277, 8724–8729.
- Gurevich, V.V.; Gurevich, E.V. How and why do GPCRs dimerize? Trends Pharmacol. Sci. 2008, 29, 234–240.
- Kufareva, I.; Salanga, C.L.; Handel, T.M. Chemokine and chemokine receptor structure and interactions: Implications for therapeutic strategies. Immunol. Cell. Biol. 2015, 93, 372–383.
- Monnier, C.; Dodé, C.; Fabre, L.; Teixeira, L.; Labesse, G.; Pin, J.P.; Hardelin, J.P.; Rondard, P. PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling activity. Hum. Mol. Genet. 2009, 18, 75–81.
- Fullone, M.R.; Lattanzi, R.; Maftei, D.; Bonaccorsi, M.C.; Miele, R. Analysis of role of aromatic residues in extracellular loop 2 of Prokineticin receptor 2 in ligand binding probed with genetically encoded photo-crosslinkers. BBA Biomembr. 2021, 1863, 183549.
- Levit, A.; Yarnitzky, T.; Wiener, A.; Meidan, R.; Niv, M.Y. Modeling of human prokineticin receptors: Interactions with novel small-molecule binders and potential off-target drugs. PLoS ONE 2011, 6, e2799.
- Lattanzi, R.; Miele, R. Non-Peptide Agonists and Antagonists of the Prokineticin Receptors. Curr. Issues Mol. Biol. 2022, 44, 6323–6332.
- Gasser, A.; Brogi, S.; Urayama, K.; Nishi, T.; Kurose, H.; Tafi, A.; Ribeiro, N.; Désaubry, L.; Nebigil, C.G. Discovery and cardioprotective effects of the first non-Peptide agonists of the G protein-coupled prokineticin receptor-1. PLoS ONE 2015, 10, e0121027.
- Bullock, C.M.; Li, J.D.; Zhou, Q.Y. Structural Determinants Required for the Bioactivities of Prokineticins and Identification of Prokineticin Receptor Antagonists. Mol. Pharmacol. 2004, 65, 582–588.
- Khusal, K.G.; Tonelli, R.R.; Mattos, E.C.; Soares, C.O.; Genova, D.B.M.; Juliano, M.A.; Urias, U.; Alves, M.J.M. Prokineticin receptor identified by phage display is an entry receptor for Trypanosoma cruzi into mammalian cells. Parasitol Res. 2015, 114, 155–165.
- Lattanzi, R.; Miele, R. Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines 2021, 9, 1648.
- Lattanzi, R.; Severini, C.; Miele, R. Prokineticin 2 in cancer-related inflammation. Cancer Lett. 2022, 546, 215838.
- Sarfati, J.; Dode, C.; Young, J. Kallmann syndrome caused bymutations in the PROK2 and PROKR2 genes: Pathophysiology and genotype-phenotype correlations. Front. Horm. Res. 2010, 39, 121–132.
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.H.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.V.; van Bruggen, N.; et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007, 450, 825–831.
- Traboulsi, W.; Brouillet, S.; Sergent, F.; Boufettal, H.; Samouh, N.; Aboussaouira, T.; Hoffmann, P.; Feige, J.J.; Benharouga, M.; Alfaidy, N. Prokineticins in central and peripheral control of human reproduction. Horm. Mol. Biol. Clin. Investig. 2015, 24, 73–81.
