Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Mucosal-associated invariant T (MAIT) cells are a distinct population of non-conventional T cells that have been preserved through evolution and possess properties of both innate and adaptive immune cells. They are activated through the recognition of antigens presented by non-polymorphic MR1 proteins or, alternately, can be stimulated by specific cytokines.
- MAIT cells
- tuberculosis
- MR1-dependent
1. Introduction
Immunity is reliant on two levels of defense; innate and adaptive immunity. The innate response, classically labeled as the ‘first line of defense’, relies on the broad recognition of microbial proteins or sugars, which upon activation, induces phagocytosis or apoptosis, cytokine release, and degranulation [1]. This generalized response can either eliminate or prevent further proliferation of the pathogen; the failure of which allows proliferation of the pathogen and subsequently provides epitopes capable of activating the adaptive response. T cells are a central part of orchestrating adaptive immunity and act by scanning the surface of antigen-presenting cells for major histocompatibility complex (MHC) molecules that present foreign polymorphic antigens, commonly in the form of antigenic peptides. These cells undergo clonal expansion in the thymus and differentiate into effector and memory T cells, allowing an immune memory against the antigen to be established. Conventional T cells play an important role in either killing infected host immune cells or activating other immune cells to regulate host immunity against a disease or to control an infection. A subset of T cells, classified as unconventional T cells, which includes invariant natural killer T (iNKT) cells, gamma-delta (γδ) T cells, and mucosal-associated invariant T (MAIT) cells, is characterized by their ability to recognize antigens presented by unconventional antigen presenting molecules and to elicit cytokine responses in a T cell receptor (TCR) independent manner. The γδ-T cells (characterized by TCRs, TRGV9-TRDV2 [Vγ9Vδ2]) recognize lipid antigens presented by CD1 molecules [2], while human iNKT cells (characterized by TCRs, Vα24-Jα18, and Vβ11) [3] recognize phospho-antigens, which are intermediates of the isoprenoid biosynthesis pathway, presented by the CD1d molecule [4].
2. MAIT Cell Development and the Microbiota
MAIT cells bridge innate and adaptive immunity [5,6]; like innate immune cells, they are the first line of defense, capable of mounting a robust immune response in an antigen-dependent/independent manner, serving as rapid responders to invasive bacterial infections, and like adaptive immune cells, attain effector memory. Single-cell RNA-sequencing comparing gene expression normally associated with innate versus adaptive immunity found that MAIT (as well as iNKT and Vδ2 T) cells expressed transcriptional profiles associated with both states of immunity, although they were transcriptionally closer to conventional T cells than to innate cells [7]. MAIT cells express the transcriptional regulators promyelocytic leukemia zinc finger (PLZF), RORγt, and T-bet, all vital for different stages of development [8,9]. These cells undergo three stages of development; initially defined as CD27−CD161− at stage 1, transitioning to CD27+CD161− in stage 2, and CD27lo/+CD161+ at stage 3 [10]. They develop in the thymus as naive cells and are postulated to be dependent on exposure to bacterial antigens of commensal microflora for further development and maturation in the periphery [10,11]. The antigens traffic from the mucosa to the thymus, which enhances MAIT cell development and expansion [12]. However, recent research has also identified MAIT cells in second trimester human fetal tissue prior to the establishment of gut microbiota [13]. Work carried out in a murine model showed that MAIT cell development occurs within a short period in the early stages of life and is strongly influenced by intestinal microbiota. This initial microbial exposure is vital in that it also determines MAIT cell abundance for life [14]. These cells range from 1–10% in blood, 2–10% in the intestine, and 20–50% in the liver [15], altogether comprising around 25% of CD8 T cells. These cells are distinguished by the markers CD161; receptors for IL-7, -12, -18, and -23; CD26; and C-C chemokine receptor 6 (CCR6), CXCR6, and CCR5. The TCR α-chain consists of TRAV1-2 with either TRAJ33/12/20 paired with variable β-chains in humans, generally TRBV6-1, TRVB 6-4 or TRBV20 [16]. The α-chain is highly evolutionarily conserved amongst mammals [17]; hence, conformational changes within the complementarity-determining region (CDR) 3β loop directs affinity for the MR1-presenting antigen [18].
A comprehensive analysis of 50 types of immune cell populations in human peripheral blood from birth to old age (75 years) showed that MAIT cells were low in the first years of life (<0.08%), increased in young children (~2.3%), peaked in adults aged 19–30 years (4.3%), and lowered again in old age (~0.9%) [19]. The same study found that CD57+ NK cells, and CD4+ and CD8+ memory T cells increased in late adulthood while dendritic cells declined. A deeper understanding of this natural shift in immune status throughout life could guide therapies to boost unconventional and conventional immune cells to protect vulnerable populations against infections.
3. Features of MAIT Cells
The main distinguishing feature of MAIT cells is their ability to recognize monomorphic MHC-1-related molecules (MR1), which present precursors from the riboflavin (RF) pathway present in many bacterial species, making them rapid responders to bacterial infection. MAIT cell-dependent activation by MR1 leads to the production of inflammatory cytokines IFN-γ, TNF-α, and IL-17 to control infection (Figure 1). In addition granzyme B and perforin are released to lyse-infected cells. The cytokines IL-12 and IL-18 [20], common signals from viral infection, activate MAIT cells in an MR1-independent manner [21], associating an innate-like activation mode to MAIT cells. In mice, MAIT cells are categorized into two subsets, MAIT17 cells, which produce IL-17 or MAIT1 cells, that produce IFNγ. Human MAIT cells, however, were found to be predominantly MAIT1 [22] with evidence that induction of the MAIT17 subset may occur during certain illnesses [23].
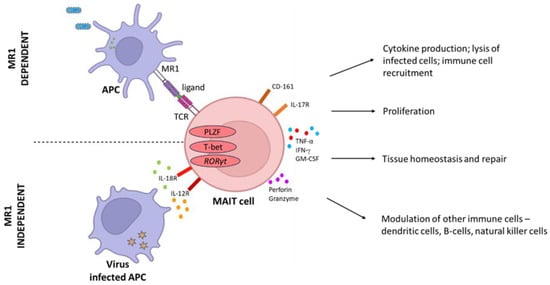
Figure 1. MAIT cell activation via MR1-dependent and MR1-independent pathways. These cells have been shown to play notable roles in response to bacterial and viral infections, autoimmune conditions, vaccine-induced immunity, tissue repair, and tissue homeostasis.
MAIT cells also modulate the activity of other immune cells to coordinate an effective adaptive immune response either through direct cell–cell contact or via signaling molecules. They play a role in the maturation of dendritic cells, the transactivation and cytotoxicity of natural killer cells [24], the reactivation and differentiation of memory B cells [25], the suppression of innate lymphoid cell proliferation [26], and the regulation of the Th1 response [27]. MAIT cell response is also mediated by costimulatory molecules of the immunoglobulin and TNF superfamilies, which is reviewed in detail by [28].
These cells are enriched in barrier tissues, locations that are most likely to come into first contact with pathogens and thus to incur a rapid inflammatory response that can cause tissue damage. A remarkable property of MAIT cells is that they express a tissue repair transcriptional profile [29,30]. Further studies showed that IL-22 and IL-17A released by the cells were linked to gut homeostasis [31]. Moreover, using a mouse skin injury model, it was demonstrated that MAIT cells produce amphiregulin, which aids in the closure of wounds, mediated in a CXCL16/CXCL6-dependent MR1-independent manner [32].
4. MR1-Bound Antigens
The earliest work in determining MAIT cell epitopes identified acetyl-6-formylpterin, a derivative of folate (vitamin B9), which although capable of binding MR1, did not activate MAIT cells [18,33]. The screening of small organic molecule and drug metabolite chemical libraries identified other molecules capable of interacting with MR1; a salicylic acid derivative, which is a by-product of the anticancer drug methotrexate, a portion of the immunosuppressant sirtinol, and the anti-inflammatory diclofenac, of which only diclofenac stimulated MAIT cell activity [34], establishing that certain drugs have the capacity to modulate MAIT cell activity. This has broader implications when considering immunotherapies to boost MAIT cell activity.
By disrupting genes involved in RF biosynthesis in Lactobacillus lactis, 5-A-RU was identified as a central intermediate in MAIT cell modulation [35]. The most potent MAIT cell activators arise from 5-A-RU from the riboflavin (RF) pathway, which interacts with methylglyoxal/glyoxal derived from the glycolysis pathway to generate the unstable pyrimidines; 5-(2-oxoethylideneamino)-6-D-ribitylaminouracil (5-OE-RU) and 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU) that stabilize upon MR1 binding [35]. It is peculiar that MR1 preferentially binds these transiently formed unstable intermediates and only weakly binds stable ligands from the RF pathway [36,37]. Other antigenic products include photolumazine-I (PLI) and -III (PLIII), again derived from 5-A-RU interacting with α-ketoglutarate [38]. The product 6,7-dimethyl-8-(d-ribityl) lumazine (RL-6,7-diMe), a precursor of RF and derivatives thereof, reduced 6-(hydroxymethyl)-8-D-ribityllumazine (rRL-6-CH2OH) and reduced 6-(hydroxymethyl)-8-(1-D-ribityl) lumazine (RL-6-Me-7-OH) are also MAIT cell activators [33]. It was demonstrated that RF-based antigens have varying degrees of potency; 5-OP-RU being the most potent, followed by 5-OE-RU and RL-6,7-diMe [39]. Following mycobacterial infection, a prominent ligand, 7,8-didemethyl-8-hydroxy-5-deazariboflavin (FO) (postulated to be formed via the interaction of 5-A-RU with a by-product of the tyrosine pathway) was identified as a MAIT cell inhibitor [38]. Moreover, RF itself was also shown to be a weak MAIT cell antagonist [38]. It is interesting that 5-A-RU can yield products that are either activators or antagonists of MAIT cell activity, depending on the by-product with which it interacts [38]. The identification of MAIT cell inhibitors and activators emanating from the mycobacterial RF pathway suggests a complex mechanism of immunoregulation. Although further studies have not shown whether Mtb may exploit this phenomenon to mitigate the activation of MAIT cells. In line with this, it would be of interest to assess whether the metabolic state of Mtb influences the ratio of antagonist/activator, skewing the immune response. An intriguing finding established that microbe metabolism is, in fact, intricately linked to MAIT cell modulation. E. coli was grown under different conditions with altered carbon sources at varying concentrations and exposure to different pH and oxygen levels to resemble host physiological conditions. Ligands extracted from bacteria at a stationary growth stage and under anaerobic conditions most potently activated MAIT cells, correlating to an increase in activating ligands [39].
Many studies have established that bacteria colonizing the human gut-microbiota produce MAIT cell ligands, giving rise to the question of why these cells are not constantly activated in the gut. It stands to reason that a symbiotic relationship must be maintained to prevent an inflammatory type response [40]. Colon biopsies showed that ~50% of MAIT cells expressed CD137+, a marker of activation. However, ~50% expressed the inhibitory receptors TIGIT+PD-1+, indicating that the cells are at different standby states. It was postulated that inhibitory molecules may be responsible for modulating this immune balance. Additionally, the activation of MAIT cells require both a TCR and cytokine signal (from pathogen recognition); hence, the absence of the secondary cytokine signals from gut microbes prevents activation.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12111343
This entry is offline, you can click here to edit this entry!
