Piperine, an active alkaloid with a wide range of therapeutic properties, including antioxidant, anti-inflammatory, and immunomodulatory effects, has garnered attention for its potential in cancer prevention and treatment. Cancer chemoprevention emboldens the use of natural and synthetic biologically active substances to prevent, inhibit, or reverse cancer progression. Chemopreventive agents have been classified into blocking agents and suppressing agents. Blocking agents impede the initiation of tumors. Suppressing agents, on the other hand, act subsequently by suppressing the transformation of initiated cells into preneoplastic and/or neoplastic cells and malignancy. Piperine exhibits a unique duality in its abilities, functioning as both a blocking and a suppressing agent in cancer prevention and therapy. This dual role allows piperine to target multiple pathways and aspects of cancer development and progression, ultimately enhancing the effectiveness of chemoprevention strategies.
- piperine
- cancer chemoprevention
- signaling pathways
1. Piperine Reduces Inflammation
2. Piperine Induces Various Cell Death Types
2.1. Apoptosis
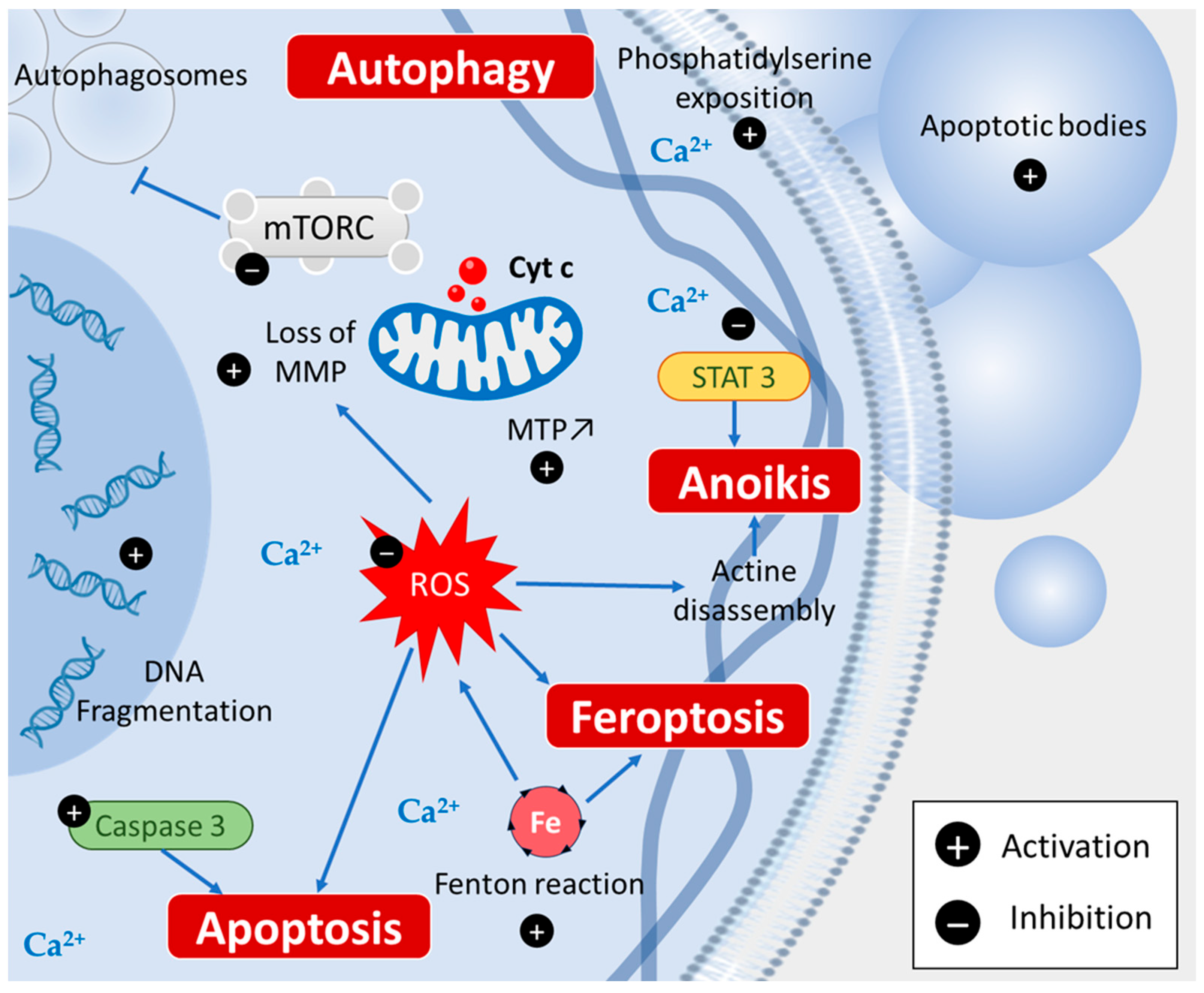
2.2. Autophagy
2.3. Ferroptosis
2.4. Anoikis
3. Piperine Inhibits Cancer Stem Cells
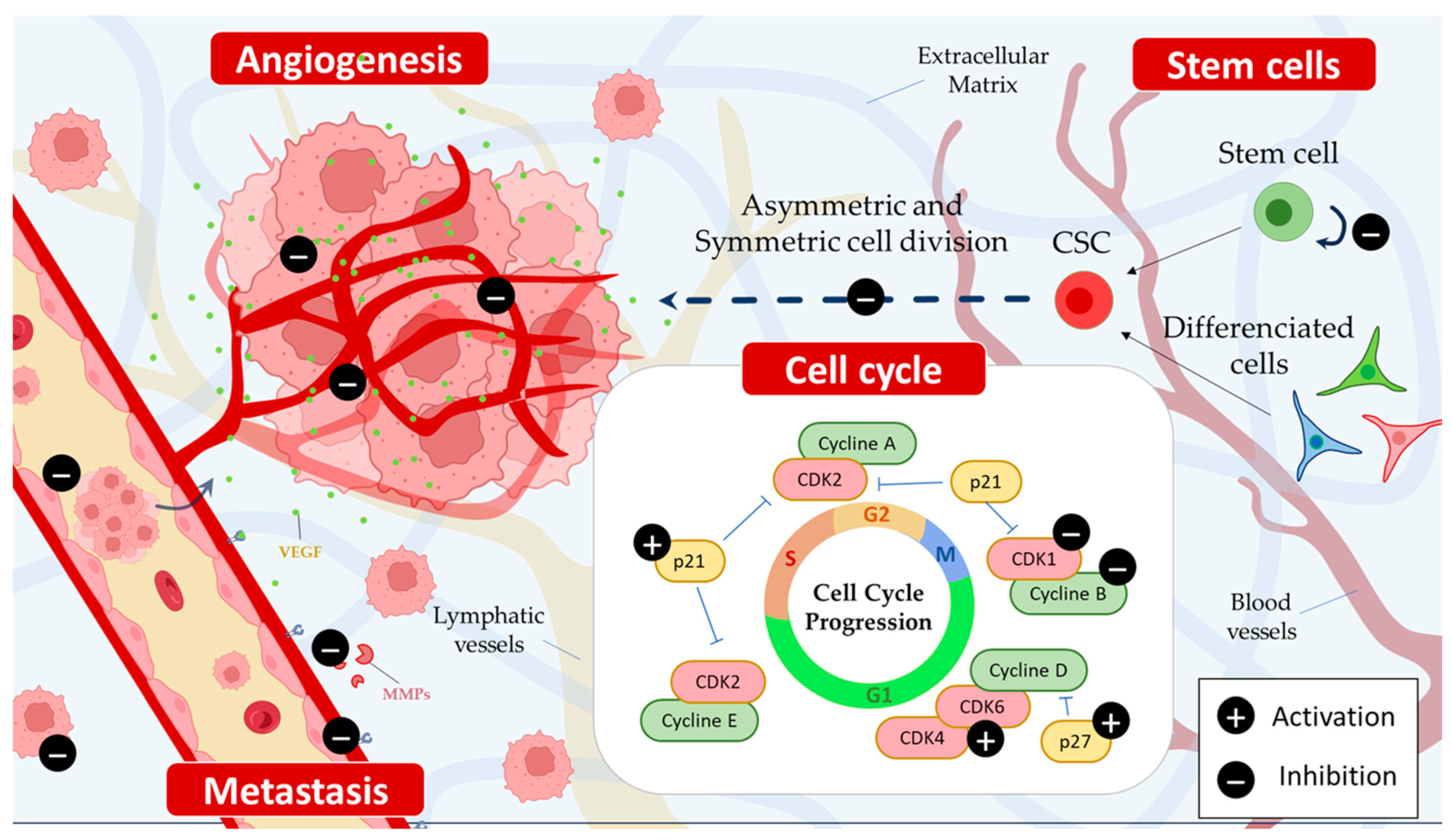
4. Piperine Induces Cell Cycle Arrest
5. Piperine Selectively Inhibits the Growth of Cancer Cells
6. Piperine Inhibits Cancer Invasion and Metastasis Process
6.1. Anti-Angiogenic Effects of Piperine
6.2. Anti-Metastatic Activity of Piperine
| Targets | Model | Mechanisms | References |
|---|---|---|---|
| In vitro | |||
| Inflammation |
|
|
[3][4][5][6] |
| Cell death |
|
|
[11][14][19][20][21][23][25][26][27] |
| Cancer stem cells |
|
|
[11][18][32][33] |
| Cell cycle |
|
|
[36][37][38][39] |
| Cancer cells growth |
|
|
[20][37][41] |
| Invasion and metastasis |
|
|
[38][43][45][46][47][48][49] |
| In vivo | |||
| Inflammation |
|
|
[9][10][11] |
| Cell death (apoptosis, autophagy) |
|
|
[15][22][50] |
| Invasion and metastasis |
|
|
[47][48] |
This entry is adapted from the peer-reviewed paper 10.3390/cancers15225488
References
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020, 263, 118607.
- Woo, H.M.; Kang, J.H.; Kawada, T.; Yoo, H.; Sung, M.K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931.
- Li, Y.; Li, K.; Hu, Y.; Xu, B.; Zhao, J. Piperine mediates LPS induced inflammatory and catabolic effects in rat intervertebral disc. Int. J. Clin. Exp. Pathol. 2015, 8, 6203–6213.
- Chuchawankul, S.; Khorana, N.; Poovorawan, Y. Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genet. Mol. Res. GMR 2012, 11, 617–627.
- Wang-Sheng, C.; Jie, A.; Jian-Jun, L.; Lan, H.; Zeng-Bao, X.; Chang-Qing, L. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int. Immunopharmacol. 2017, 42, 44–48.
- Zazeri, G.; Povinelli, A.P.R.; Le Duff, C.S.; Tang, B.; Cornelio, M.L.; Jones, A.M. Synthesis and Spectroscopic Analysis of Piperine- and Piperlongumine-Inspired Natural Product Scaffolds and Their Molecular Docking with IL-1β and NF-κB Proteins. Molecules 2020, 25, 2841.
- Wang, L.; Yin, Q.; Liu, C.; Tang, Y.; Sun, C.; Zhuang, J. Nanoformulations of Ursolic Acid: A Modern Natural Anticancer Molecule. Front. Pharmacol. 2021, 12, 706121.
- Kumar, S.; Malhotra, S.; Prasad, A.K.; Van der Eycken, E.V.; Bracke, M.E.; Stetler-Stevenson, W.G.; Parmar, V.S.; Ghosh, B. Anti-inflammatory and antioxidant properties of Piper species: A perspective from screening to molecular mechanisms. Curr. Top. Med. Chem. 2015, 15, 886–893.
- Sireeratawong, S.; Itharat, A.; Lerdvuthisopon, N.; Piyabhan, P.; Khonsung, P.; Boonraeng, S.; Jaijoy, K. Anti-Inflammatory, Analgesic, and Antipyretic Activities of the Ethanol Extract of Piper interruptum Opiz. and Piper chaba Linn. ISRN Pharmacol. 2012, 2012, 480265.
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.-J.; Lim, S.-J.; Kim, J.Y.; Yang, H.-I.; Yoo, M.C.; Hahm, D.-H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49.
- Tawani, A.; Amanullah, A.; Mishra, A.; Kumar, A. Evidences for Piperine inhibiting cancer by targeting human G-quadruplex DNA sequences. Sci. Rep. 2016, 6, 39239.
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516.
- Fitzgerald, M.-C.; O’Halloran, P.J.; Connolly, N.M.C.; Murphy, B.M. Targeting the apoptosis pathway to treat tumours of the paediatric nervous system. Cell Death Dis. 2022, 13, 460.
- Jafri, A.; Siddiqui, S.; Rais, J.; Ahmad, M.S.; Kumar, S.; Jafar, T.; Afzal, M.; Arshad, M. Induction of apoptosis by piperine in human cervical adenocarcinoma via ROS mediated mitochondrial pathway and caspase-3 activation. EXCLI J. 2019, 18, 154–164.
- Qi, Y.; Yao, L.; Liu, J.; Wang, W. Piperine improves the sensitivity of osteosarcoma cells to doxorubicin by inducing apoptosis and inhibiting the PI3K/AKT/GSK-3β pathway. J. Orthop. Surg. Res. 2023, 18, 180.
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650.
- Chen, H.Y.; White, E. Role of autophagy in cancer prevention. Cancer Prev. Res. 2011, 4, 973–983.
- Alvarez-Meythaler, J.G.; Garcia-Mayea, Y.; Mir, C.; Kondoh, H.; ME, L.L. Autophagy Takes Center Stage as a Possible Cancer Hallmark. Front. Oncol. 2020, 10, 586069.
- Kaur, H.; He, B.; Zhang, C.; Rodriguez, E.; Hage, D.S.; Moreau, R. Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: Implications for the inhibition of protein synthesis and TNFα signaling. J. Nutr. Biochem. 2018, 57, 276–286.
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 424–430.
- Zhu, P.; Qian, J.; Xu, Z.; Meng, C.; Liu, J.; Shan, W.; Zhu, W.; Wang, Y.; Yang, Y.; Zhang, W.; et al. Piperlonguminine and Piperine Analogues as TrxR Inhibitors that Promote ROS and Autophagy and Regulate p38 and Akt/mTOR Signaling. J. Nat. Prod. 2020, 83, 3041–3049.
- Han, E.J.; Choi, E.Y.; Jeon, S.J.; Lee, S.W.; Moon, J.M.; Jung, S.H.; Jung, J.Y. Piperine Induces Apoptosis and Autophagy in HSC-3 Human Oral Cancer Cells by Regulating PI3K Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 13949.
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364.
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10.
- Mittal, R.; Gupta, R.L. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274.
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022.
- Fofaria, N.M.; Srivastava, S.K. STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis 2015, 36, 142–150.
- Economopoulou, P.; Kaklamani, V.G.; Siziopikou, K. The role of cancer stem cells in breast cancer initiation and progression: Potential cancer stem cell-directed therapies. Oncologist 2012, 17, 1394–1401.
- Scarpa, E.S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8.
- de Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681.
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Anil Kumar, N.V.; Salehi, B.; Cho, W.C.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A Review of Its Bioactivity and Studies. Appl. Sci. 2019, 9, 4270.
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54.
- Samiry, I.; Pinon, A.; Limami, Y.; Rais, S.; Zaid, Y.; Oudghiri, M.; Liagre, B.; Mtairag, E.M. Antitumoral activity of Caralluma europaea on colorectal and prostate cancer cell lines. J. Toxicol. Environ. Health Part A 2023, 86, 230–240.
- Fofaria, N.M.; Kim, S.H.; Srivastava, S.K. Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation. PLoS ONE 2014, 9, e94298.
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58.
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140.
- Pressete, C.G.; Viegas, F.P.; Campos, T.G.; Caixeta, E.S.; Hanemann, J.A.; Ferreira-Silva, G.Á.; Zavan, B.; Aissa, A.F.; Miyazawa, M.; Viegas, C.; et al. Piperine–Chlorogenic Acid Hybrid Inhibits the Proliferation of the SK-MEL-147 Melanoma Cells by Modulating Mitotic Kinases. Pharmaceuticals 2023, 16, 145.
- Suter, T.M.; Ewer, M.S. Cancer drugs and the heart: Importance and management. Eur. Heart J. 2013, 34, 1102–1111.
- Gunasekaran, V.; Elangovan, K.; Niranjali Devaraj, S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 105, 106–118.
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.; Oon, C.E. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina 2018, 54, 8.
- Tobelem, G. Tumor angiogenesis. Nouv. Rev. Fr. D’hematol. 1990, 32, 405–406.
- Doucette, C.D.; Hilchie, A.L.; Liwski, R.; Hoskin, D.W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 2013, 24, 231–239.
- Qi, Y.B.; Yang, W.; Si, M.; Nie, L. Wnt/β-catenin signaling modulates piperine-mediated antitumor effects on human osteosarcoma cells. Mol. Med. Rep. 2020, 21, 2202–2208.
- Zahra, Z.; Tina Nayerpour, D.; Armaghan, L.; Zakieh Sadat, S.; Mohammad, P.; Fahimeh, H.; Vajiheh, N.; Omid, A.; Mojtaba, A.; Parisa, K. The Effect of Piperine on MMP-9, VEGF, and E-cadherin Expression in Breast Cancer MCF-7 Cell Line. Basic Clin. Cancer Res. 2020, 12, 112–119.
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523–530.
- Gasser, A.B.; Depierre, D.; Courvoisier, B. Total urinary and free serum hydroxyproline in metastatic bone disease. Br. J. Cancer 1979, 39, 280–283.
- Lin, M.T.; Lin, B.R.; Chang, C.C.; Chu, C.Y.; Su, H.J.; Chen, S.T.; Jeng, Y.M.; Kuo, M.L. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int. J. Cancer 2007, 120, 2600–2608.
- Srivastava, S.; Dewangan, J.; Mishra, S.; Divakar, A.; Chaturvedi, S.; Wahajuddin, M.; Kumar, S.; Rath, S.K. Piperine and Celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/β-catenin signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2021, 84, 153484.
