Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
CircRNAs are a recently discovered class of ncRNA molecules. They are formed during the process of RNA transcript maturation. Structurally, circRNAs are covalently closed by a connection between a downstream donor and upstream acceptor RNA splice sites linked by a phosphodiester bond.
- circular RNAs (circRNAs)
- hepatocellular carcinoma (HCC)
- immunotherapy
- cytotoxic T lymphocytes
1. What Are CircRNAs?
CircRNAs are a recently discovered class of ncRNA molecules. They are formed during the process of RNA transcript maturation. Structurally, circRNAs are covalently closed by a connection between a downstream donor and upstream acceptor RNA splice sites linked by a phosphodiester bond. CircRNAs were previously regarded as splicing junk but are now recognized as functional RNA molecules [31]. They have expression patterns that are particular to different tissues and cell types, and they are produced from a wide variety of genes [51]. It is noteworthy that circRNAs are implicated in biological processes that contribute to the development and spread of cancer [52,53].
Additionally, due to their circular shape and resistance to exoribonuclease activity, circRNAs have longer half-lives than their parental linear counterparts, making it possible to detect them even when produced at low levels [41,54,55]. For instance, exonic circRNAs are thought to be extremely stable in cells, with most circRNAs showing half-lives of over 48 h, as opposed to an average mRNA half-life of 10 h [54,56]. These characteristics imply that circRNAs could serve as useful biomarkers for the diagnosis and prognosis of cancer patients, as previously described in [41,50].
2. Biogenesis of CircRNAs
Recent research has shown that “back-splicing”, a type of pre-mRNA splicing, is responsible for the production of circRNAs [57]. CircRNAs have a distinctive closed-loop structure, created by linking a downstream 5′ splice donor site and an upstream 3′ splice acceptor site, in contrast to conventional pre-mRNAs with 5′ caps and 3′ polyadenylated tails [58,59].
CircRNAs are primarily categorized into four types [60,61] based on the origin of their genomic regions: exonic circRNAs (EcircRNAs), retained-intron or exonic-intronic circRNAs (EIcircRNAs), intronic circRNAs (ciRNAs), and tRNA intronic circRNAs (tricRNAs) [62]. Over 80% of the circRNAs that have been discovered are EcircRNAs, and these circRNAs are mostly found in the cytoplasm [63,64]. As EcircRNAs sponge miRNAs and/or interact with RBPs, several studies have shown that EcircRNAs play significant roles in modulating the genetic expression of several coding transcripts [65,66]. EIciRNAs and ciRNAs, which compose a minor portion of circRNAs compared to EcircRNAs, are mostly found in the nucleus and, thus, can control the expression of their parental mRNAs, as shown in Figure 1 [57]. The following section will cover the four associated biogenesis mechanisms of circRNAs.
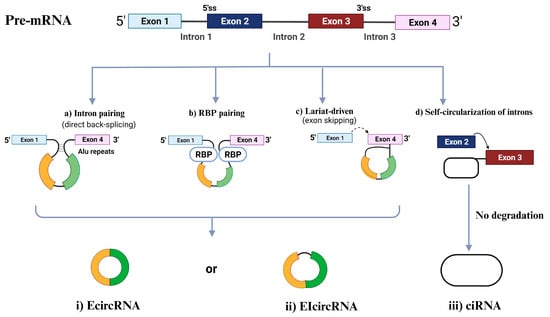
Figure 1. Circular RNA (circRNA) biogenesis. Schematic presentation of the different mechanisms of circRNAs biogenesis: intron painting (a), RBP pairing (b), Lariat driven (c), and the self-circularization of introns (d). ciRNA, intronic circRNA; EcircRNA, exonic circRNA; EIcircRNAs, exon-intron circRNA; RBP, RNA-binding protein; ss, splice site.
2.1. Intron Pairing-Driven Circularization
The most frequent circularization process of EcircRNA and EIciRNA involves “direct back-splicing”, also known as intron-pairing-driven circularization, in which a particular pre-mRNA with ALU repeats is sheared to generate an EcircRNA or an EIciRNA following reverse-base complementary pairing [56].
2.2. RBP-Induced Circularization
RBPs, which are thought to be trans-acting factors and include Quaking (QKI), Muscleblind (MBL), and Fused-in Sarcoma (FUS), may promote circularization by bridging similar intronic sequences [67]. The 3′ and 5′ termini of circularized exons can be brought into closer proximity through the dimerization of RBPs. This dimerization process also facilitates splicing by engaging with the sequences both upstream and downstream of the circularized exons [68].
2.3. Lariat-Induced Circularization Driven by Spliceosomes
Lariat-driven circularization, also known as the exon-skipping mechanism, occurs as pre-mRNA partially folds during transcription. This folding brings the 5′ splice site (donor site) of the upstream intron close to the 3′ splice site (receptor site) of the downstream intron, forming a circRNA through back-splicing within the folded region. The remaining exons then combine to create a linear mRNA [56]. Moreover, back splicing can occur post-transcriptionally or co-transcriptionally, involving either a single exon or multiple exons with intervening introns [69].
2.2.4. Self-Circularization of Introns
Intron self-circularization occurs when a pre-RNA contains 7 nucleotides (nt) of guanine (G) and uracil (U)-rich sequence close to one exon and an 11 nt cytosine (C)-rich sequence close to another exon. This allows the introns to avoid branching and degradation during splicing, resulting in a stable intronic lariat structure [57].
3. Functional Roles of CircRNAs
CircRNAs typically function as regulatory ncRNA molecules, either directly by controlling gene transcription or indirectly by modifying other regulators, such as proteins and miRNAs. Further, the term “regulatory coding RNAs” refers to a subset of circRNAs that encode short functional peptides, as shown in Figure 2 and described below [53].
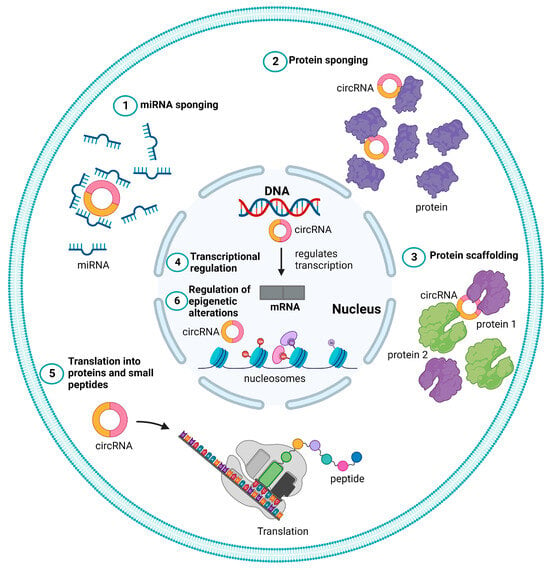
Figure 2. Different functional roles of circular RNAs (circRNAs). Schematic representation of different mechanisms of action of circRNAs represented as (1) microRNA (miRNA) sponge, (2) protein sponge or decoy (3) protein scaffolding, (4) transcriptional regulation, (5) translation to proteins, and peptide (6) regulation of epigenetic alterations.
3.1. miRNA Sponge
Some circRNAs may behave as miRNA sponges or sequesters because they include well-conserved canonical miRNA response elements (MREs) [70,71,72]. Some circRNAs that act as miRNA sponges can positively or adversely affect the expression of the corresponding targeted genes. Cerebellar degeneration-related protein 1 antisense (CDR1-AS or ciRS-7), a well-studied circRNA, has been linked to a variety of malignancies, including HCC and gastric cancer, as well as sponges miR-7 in embryonic zebrafish [73,74,75]. Indeed, a growing body of research has shown that the circRNA-miRNA-mRNA regulatory network may have significant effects on several diseases, including HCC [76,77].
For instance, circ-ZNF609 increases the expression of the myocyte-specific enhancer factor 2A (MEF2A), which improves vascular endothelial dysfunction by acting as an endogenous miR-615-5p sponge to decrease miR-615-5p activity [78].
According to Zhong et al., circ-MYLK can ease the inhibition of its target vascular endothelial growth factor A (VEGFA), a crucial component of the VEGFA/VEGFR2/RAS/MAPK1 signaling pathway, in addition to being associated with the stage and grade of bladder carcinoma [80]. By sequestering miR-143 and increasing the production of its target BCL2, increased levels of circ-UBAP2 stimulate the proliferation of osteosarcoma cells while preventing apoptosis both in vitro and in vivo [81]. Similarly, circ-ABCB10 has been shown by Liang et al. to sponge miR-1271, promoting proliferation and inhibiting the apoptosis of breast cancer cells [82].
3.2. Protein Sponge or Decoy
CircRNAs can also bind and sequester proteins using their protein-binding sites, functioning as an antagonist to impede their physiological function [83]. RBPs are one of the most common protein classes that can bind to circRNAs. For instance, circ-TNPO3 functions as a protein decoy for the insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) to inhibit the capacity of gastric cancer cells to proliferate [84]. The expression of MYC proto-oncogene, as well as bHLH transcription factor (MYC) and its target, snail family transcriptional repressor 1 (SNAI1), is inhibited when circ-TNPO3 sequesters IGF2BP3, which reduces the ability of gastric cancer cells to proliferate and metastasize [84]. It was also reported that circ-SIRT1 binds to the eukaryotic translation initiation factor 4A3 (EIF4A3) in colorectal cancer cell lines, preventing its inhibitory impact on epithelial–mesenchymal transition and encouraging the proliferation and invasion of colorectal cancer cell lines [86].
CircRNAs can also decoy proteins by attaching themselves to cellular proteins and changing how they normally carry out their physiological functions [44,87]. Circ0000079 (ciR79) inhibits the induction of fragile X-related 1 (FXR1) protein and prevents its complexation with protein kinase C iota (PRKCI), thus preventing the FXR1/PRKCI-mediated phosphorylation of glycogen synthesis kinase 3β (GSK3B) and activator protein 1 (AP-1), suppressing SNAI1 protein levels and hindering non-small cell lung cancer growth [88].
3.3. Protein Scaffolding
CircRNAs with enzyme and substrate binding sites are believed to serve as scaffolds that help two or more proteins to come into proximity and interact. CircFoxo3, which includes binding sites for MDM2 and p53, serves as an indicative case of this observation. In order to support the idea that circFoxo3 can serve as a protein scaffold, the mutation of these binding sites or circRNA silencing reduced the amount of p53 that an MDM2 antibody could pull down. Further research revealed that circFoxo3 promoted the ubiquitination of p53 by MDM2, which is then destroyed by the proteasome. Additionally, circACC1 forms a ternary complex with the regulatory β and γ subunits of AMP-activated protein kinase (AMPK), stabilizing and enhancing the enzymatic activity of the AMPK holoenzyme [89]. More circRNAs acting as scaffolds are expected to be identified in the future because of the longer half-lives of circRNAs [90].
3.4. Transcriptional Regulation
CircSEP3 derived from SEP3 exon 6 enhances the abundance of homologous exon 6-skipped variant by attaching to the host DNA locus and creating an RNA-DNA hybrid or R-loop, which causes transcription to pause and splicing factor recruitment [91]. Similarly, circSMARCA5 induces the expression of the shortened non-functional isoform by causing transcriptional termination of the SWI/SNF-related, matrix-associated, and actin-dependent regulator of chromatin, subfamily a, member 5 (SMARCA5) at exon 15 through R-loop formation [92]. EIciRNAs can interact with the U1 small nuclear ribonucleoprotein to increase the expression of parental genes through RNA-RNA interactions with snRNA molecules [93]. As lariats evade debranching, circRNAs can amass at their formation sites and enhance the activity of RNA polymerase II, resulting in the increased expression of the respective genes [57].
3.5. Translation to Proteins and Peptides
The ability of circRNAs to undergo translation was originally discovered by Pamudurti et al. in 2017 [94]. According to bioinformatics studies, some circRNAs contain an open reading frame (ORF), which indicates that they can be translated. Ribosome profiling, which can sequence ribosome-covered RNAs to track translation in vivo, has shown convincing evidence that some circRNAs comprising internal ribosome entry sites (IRES) are translated based on an IRES-dependent mechanism [94], whereas other circRNAs are translated independently of IRES elements. The translation of circSHPRH into the SNF2 histone linker PHD RING helicase (SHPRH)-146aa protein was demonstrated to be IRES-dependent. It was discovered that SHPRH-146aa is a tumor suppressor protein that guards against the degradation of the SHPRH full-length protein. Therefore, incorrect circSHPRH translation affects tumor malignancy [95].
Other circRNAs have also been discovered to encode functional peptides and proteins that have tumor-promoting or -suppressing properties [95,96,97]. Finally, certain circRNAs can encode peptides without the need for IRES. Protein translation is made easier, for instance, by the m6A modification. The m6A reader protein YTH N6-methyladenosine RNA binding protein F3 (YTHDF3) interacts with translation initiation factors to start protein synthesis by binding to circRNAs that include m6A modification sites [98,99,100].
3.6. Regulation of Epigenetic Alterations
Cancer commonly exhibits abnormal DNA methylation and histone alterations that are linked to the epigenetic regulation of gene expression [101,102]. It has been discovered that certain circRNAs control these epigenetic changes. According to Chen et al. [103], circFECR1 significantly reduced the amount of CpG DNA methylation in the promoter of Fli-1 proto-oncogene, ETS transcription factor (FLI1), which epigenetically activated FLI1. Through binding to the DNA methyltransferase 1 (DNMT1) promoter, circFECR1 has been shown to suppress the transcription of DNMT1, a crucial methyltransferase enzyme necessary for the upkeep of DNA methylation. Additionally, tet methylcytosine dioxygenase 1 (TET1) DNA demethylase might be attracted by circFECR1 to the FLI1 promoter and cause DNA demethylation. A component of polycomb-repressive complex 2 (PRC2), as an enhancer of zeste homolog 2 (EZH2), serves as an H3K27 methyltransferase and controls histone methylation [104,105]. Moreover, hsa-circ0020123 can upregulate EZH2 and zinc finger E-box binding homeobox 1 (ZEB1) using sponging miR-144, while circBCRC4 can enhance the expression of EZH2 by interacting with miR-101 [106,107].
4. Involvement of circRNAs in HCC Tumor Development and Progression
It has been reported that circRNAs have a fundamental role in the etiology of several human diseases, including several oncological conditions [108]. According to earlier investigations, circRNAs are thought to be important to the onset, development, and growth of HCC. For instance, circ0008450 induces HCC cellular proliferation, invasion, and migration and reduces apoptosis caused by sponging miR-548 [109]. Additionally, circRNA-104718 can similarly enhance HCC cellular proliferation, invasion, and migration and inhibit apoptosis by regulating the miRNA-218-5p/TXNDC5 axis [110]. The circular RNA hsa_circ_0078710 enhances cell proliferation by sequestering miR-31, resulting in the upregulation of histone deacetylase 2 (HDAC2) and cyclin-dependent kinase 2 (CDK2) expression [111]. Circ-ZEB1.33 facilitates the proliferation of HCC cells by modulating the miR-200a-3p/CDK6axis [112]. Hsa_circ_0016788 expedites HCC growth through the regulation of miR-481 and its downstream target cyclin-dependent kinase 4 (CDK4) [113]. Furthermore, hsa_circ_0091581 promotes the proliferation of HCC cells by elevating MYC levels, acting as a sponge for miR-526b [114]. Additionally, circBACH1 directly interacts with the RNA binding protein HuR, promoting the cytoplasmic accumulation of HuR, thus leading to decreased cyclin-dependent kinase inhibitor 1B (CDKN1B) expression [115], which influences cell cycle progression.
Pu et al. observed a significant increase in hsa_circ_0000092 expression in HCC tissues and cell lines. Depleting hsa_circ_0000092 inhibited HCC cell proliferation, migration, invasion, and angiogenesis in vitro and in vivo. This circRNA promotes HCC angiogenesis by acting as a miR-338-3p sponge, leading to increased expression of Jupiter microtubule-associated homolog 1 (JPT1), matrix metallopeptidase 9 (MMP9), and VEGFA [116]
Recent research highlights the pivotal roles of circRNAs in the regulation of apoptotic mechanisms within HCC. Specifically, these circRNAs target key components involved in both anti-apoptotic and pro-apoptotic signaling pathways. Notably, circ-BIRC6 exhibits significant overexpression in HCC tissue samples and correlates with the overall survival of HCC patients. Silencing circ-BIRC6 expression effectively enhances apoptosis in HCC cells by modulating BCL2 apoptosis regulator (BCL2) levels through the sequestration of miR-3918 [117]. Moreover, circ-0051443 displays reduced expression in HCC tissues and plasma. Exosomal circ-0051443 exerts a suppressive influence on the biological behaviors of HCC cells, primarily by promoting apoptosis through the interaction with miR-331-3p and the regulation of BCL2 antagonist/killer 1 (BAK1) [118]. On the other hand, certain circRNAs have inhibitory influences on the development of HCC. For instance, circADAMTS14 regulates miR-572/RCAN1, leading to the abrogation of HCC cellular hallmarks and inducing HCC cellular apoptosis machinery [119]; circRNA-5692 has a similar inhibitory impact on HCC progression by controlling the miR-328-5p/DAB2IP axis [120]. Table 1 represents a comprehensive list of all characterized oncogenic and tumor suppressor circRNAs in HCC.
Table 1. Oncogenic and tumor suppressor circular RNAs in hepatocellular carcinoma (HCC).
| Circular RNA | Class | Molecular Targets | In Vitro/In Vivo/Ex Vivo Model | References |
|---|---|---|---|---|
| SCD-circRNA2 | Oncogenic | MAPK1, RBM3 | Huh7 HepG2 HCT-15 NCI-N87 |
[121] |
| circRHOT1 | Oncogenic | NR2F6 | HCC Tissues | [122] |
| circ-100338 | Oncogenic | MMP2, MMP9 | Hep3B HLE Huh7 BEL7402 SMCC7721 MHCC97L MHCC97H HCCLM3 HCCLM6 |
[123] |
| circ-0000092 | Oncogenic | miR-338-3p | Hep3B LM3 MHCC97L SK-hep1 HepG2 |
[116] |
| circPRMT5 | Oncogenic | miR-188-5p/HK2 axis | HCC tissues HCCLM3 SNU-387 |
[124] |
| circMAT2B | Oncogenic | PKM2 | HepG2 Huh7 SMMC-772 MHCC-97L MHCC-97H |
[125] |
| circASAP1 | Oncogenic | MAPK1 | MHCC97L MHCC97H HCCLM3 | [126] |
| circβ-catenin | Oncogenic | β-catenin | Huh7 | [97] |
| circUHRF1 | Oncogenic | UHRF1 | HepG2 HCCLM3 SMMC-7721 Huh 7 PLC/PRF/5 Hep3B |
[127] |
| circ-CDYL | Oncogenic | PI3K-AKT-MTORC1/β-catenin and NOTCH2 | HCCLM SMMC7721 |
[128,129] |
| circ-0046600 | Oncogenic | HIF-1α | HepG2 SK-HEP-1 |
[130] |
| hsa_circ_0101432 | Oncogenic | MAPK1 | Huh-7 SK-HEP-1 HepG2 HLE |
[131] |
| circMAN2B2 | Oncogenic | MAPK1 | HL-7702 | [132] |
| circPTGR1 | Oncogenic | MET | HepG2 97L LM3 |
[133] |
| circ-DB | Oncogenic | miR-34a, and USP7 | HepG2 Hepa 1-6 3T3L1 |
[134] |
| circRNA Cdr1as | Oncogenic | AFP | SMMC-7721 Bel-7402 HepG2 Hep3B Huh-7 HB611 |
[135] |
| circRNA PVT1 | Oncogenic | miR-203/HOXD3 pathway | SMMC-7721 Huh-7 | [136] |
| circPVT1 | Oncogenic | TRIM23/miR-377 axis | SNU-387 Huh-7 |
[137] |
| hsa_circ_0008450 | Oncogenic | EZH2 | SMMC7721 Sk-Hep-1 HepG2 Huh-7 HCCLM3 |
[138] |
| circ_0008450 | Oncogenic | miR-548 | HepG2 Huh-7, SMMC7721 Sk-Hep-1 HCCLM3 |
[109] |
| hsa_circRNA_103809 | Oncogenic | miR-377-3p/FGFR1/MAPK1 axis | MHCC97L Huh7 SK-HEP-1 Hep3B HCCLM3 |
[139] |
| circRNA-104718 | Oncogenic | miR-218-5p/TXNDC5 | HCC nude mice model | [110] |
| circMYLK | Oncogenic | miR-362-3p/Rab23 | Huh7 Hep3B |
[140] |
| circ-ZNF652 | Oncogenic | miR-29a-3p/GUCD1 Axis | SNU-387 Huh-7 |
[141] |
| circ_0000267 | Oncogenic | miR-646 | HepG2 Huh-7 SMMC7721 Sk-Hep-1 HCCLM3 |
[142] |
| circ-FOXP1 | Oncogenic | miR-875-3p, miR-421, SOX9 factor | SNU-387 HepG2 Hep3B Huh7 SMMC-7721 HCCLM3 |
[143] |
| circRNA_104075 | Oncogenic | YAP-dependent tumorigenesis through regulating HNF4a | Bel-7402 SMMC-7721 Huh7 HepG2 Hep1 Bel-7404 THLE-3 HL-7702 |
[144] |
| hsa_circ_101280 | Oncogenic | miR-375/JAK2 | HepG2 SNU-398 |
[145] |
| circRNA-101368 | Oncogenic | HMGB1/RAGE | HCCLM3 HepG2 | [146] |
| circ-ZEB1.33 | Oncogenic | miR-200a-3p-CDK6 | 97H Huh7 HepG2 SNU423 SNU475 L02 |
[112] |
| circFBLIM1 | Oncogenic | miR-346 | HCC tissues HCC mouse model |
[147] |
| hsa_circ_0103809 | Oncogenic | miR-490-5p/SOX2 signaling pathway | MHCC97H HepG2 Huh7 SMMC7721 SK-Hep1 |
[148] |
| hsa_circ_0016788 | Oncogenic | miR-486/CDK4 | HepG2 Hep3B Huh7 HCCLM3 MHCC97L |
[113] |
| hsa_circRBM23 | Oncogenic | miR-138 | HCC tissues HepG2 Huh7 Bel-7402 |
[149] |
| hsa_circ_0005075 | Oncogenic | miR-431 | SMMC-7721 | [150] |
| circABCC2 | Oncogenic | miR-665 | HepG2 Bel-7402 MHCC97H |
[151] |
| hsa_circ_100338 | Oncogenic | MTOR signaling pathway | SMMC7721 Bel-7402 Hep3B |
[152] |
| circ_0091581 | Oncogenic | miR-591/FOSL2 axis | THLE-2 | [153] |
| circPCNX | Oncogenic | miR-506 | HL-7702 SMMC-7721 HuH-7 Hep3B HepG2 |
[154] |
| hsa_circ_0056836 | Oncogenic | miR-766-3p/FOSL2 axis | Huh7 HepG2 SNU449 SK-HEP-1 |
[155] |
| circ- HOMER1 | Oncogenic | miR-1322 on CXCL6 | Sk-Hep-1 SMMC7721 HCCLM3 Huh-7 HepG2 |
[156] |
| circ_0091579 | Oncogenic | miR-136-5p/TRIM27 miR-1270/YAP1 miR-1225/PLCB1 |
HCCLM3 MHCC97H Huh-7 |
[157,158,159] |
| circ_0001955 | Oncogenic | miR-516a-5p miR-646 miR-145-5p/NRAS |
Huh-7 HepG2 SMMC-7721 Bel-7402 Hep-3B |
[160,161,162] |
| circTRIM33-12 | Tumor suppressor | miR-191 | HCC tissues MHCC97-L MHCC97-H LM3 |
[163] |
| circHIAT1 | Tumor suppressor | PTEN | Hep3B SMMC-7721 HepG2 LM3 |
[164] |
| circLARP4 | Tumor suppressor | miR-761/RUNX3/p53/CDKN1A pathway | Huh7 Hep3B SMMC7721 HepG2 |
[165] |
| circMTO1 | Tumor suppressor | miR-9-5p/NOX4 axis | HepG2 Hep3B |
[166] |
| circITCH | Tumor suppressor | miR-184 | Huh7 HCCLM3 SMMC-7721 MHCC97H HepG2 |
[167] |
| circFBXW4 | Tumor suppressor | miR-18b-3p/FBXW7 axis | LX-2 | [168] |
| mmu_circ_34116 | Tumor suppressor | miR-661/PTPN11 | HepG2, SNU449 | [169] |
| hsa_circ_0007874/cMTO1 | Tumor suppressor | miR-338-5p | HCCLM3 MHCC97-L Hep3B SMMC-7721 Huh7 Bel-7402 MHCC97-H |
[170] |
| circ608 | Tumor suppressor | miR-222/PINK1 | Primary hepatic stellate cells (PHSCs) from C57BL/6 mice | [171] |
| hsa_circ_0070963 | Tumor suppressor | miR-223-3p LEMD3 |
LX2 | [172] |
| hsa_circ_0004018 | Tumor suppressor | miR-626/DKK3 | Huh7 Bel7402 SNU182 Hep3B SNU449 |
[173] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms242216484
This entry is offline, you can click here to edit this entry!
