Edible insects are abundant in protein content, encompassing all essential amino acids required for a balanced diet. This is a viable alternative protein source for feeding options to the expanding world population. It has significant potential for enhancing food security, offering a sustainable and environmentally conscious alternative to conventional protein sources.
- insects
- protein
- bioactive peptides
- alternative protein
- functional properties
- kosher
- Halal
1. Edible Insects
| Insect | Chicken | Pig | Cow | |
|---|---|---|---|---|
| Greenhouse gases released per kg of live weight, g | 2 | NA | 1130 | 2850 |
| Feed required per kg of live weight, kg | 1.7 | 2.5 | 5 | 10 |
| Land required per g of protein | 18 | 51 | 63 | 254 |
| Water required per g of protein, liter | 23 | 34 | 57 | 112 |
2. Insects Nutritional Value of Edible Insects
2.1. Protein Content of Edible Insects
| Insect’s Name | Protein Content Range (%) | Reference |
|---|---|---|
| Larvae | 26–45 | [27,28,29,30,31] |
| Cricket, Grasshoppers, Locusts | 6–77 | [27,28] |
| Grasshopper | 20–56 | [28,29] |
| Beetles, Grubes | 8–69 | [28,32,33] |
| Termites | 20–43 | [28,29,33] |
| Bees, Ants | 5–66 | [28,33] |
| Dragonfly | 26–54 | [34] |
| Cockroaches | 43–66 | [33] |
| Flies | 35–64 | [33] |
| True Bugs | 27–71 | [33] |
| Butterflies, Moths | 18–60 | [33] |
| Dragonflies, Damselflies | 54–56 | [33] |
2.2. Amino Acid Composition
| Source | Lys * | His ** | Arg ** | Asp | Thr * | Ser | Glu | Pro | Gly | Ala | Met * | Cys | Val * | Ile * | Leu * | Phe * | Tyr ** | Trp * | AAS *** (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zonocerus variegatus | 48.4 | 39.2 | 60.6 | 81.9 | 30.7 | 46.7 | 133.7 | 43.0 | 44.9 | 36.6 | 18.9 | 6.5 | 35.4 | 36.7 | 50.6 | 30.5 | 25.3 | - | 66.4 | [43] |
| Periplaneta americana L.a | 40.0 | 20.0 | 51.0 | - | 36.0 | 45.0 | 130 | 65.0 | 71.0 | 61.0 | 36.0 | 20.0 | 65.0 | 31.0 | 56.0 | 31.0 | 69.0 | 6.0 | 76.3 | [44] |
| Rhynchophoris phoenicis (larvae) | 45.0 | 38.9 | 79.2 | 30.6 | 39.0 | 156.0 | 50.1 | 47.2 | 52.5 | 19.7 | 20.2 | 35.0 | 39 | 54.2 | 47.5 | 29.0 | - | 71.9 | [45] | |
| Sciphophorus acupunctatus (larvae) | 53.5 | 14.7 | 44.0 | - | 40.4 | - | - | - | - | - | 20.2 | 26.7 | 62.0 | 48.2 | 78.2 | 46.1 | 63.5 | 8.1 | 83.4 | [46,47] |
| Ephydra hians (larvae) | 55.0 | 10.0 | - | 49.0 | - | - | - | - | - | 19.0 | - | 61.0 | 40.0 | 74.0 | 54.0 | 51.0 | 7.1 | 74.3 | [48] | |
| Hoplophorion monograma | 55.0 | 15.0 | - | 45.0 | - | - | - | - | - | 19.0 | - | 74.0 | 41.0 | 77.0 | 47.0 | 90.0 | 9.6 | 84.1 | [48] | |
| Atta mexicanah | 49.0 | 25.0 | - | 43.0 | - | - | - | - | - | 34.0 | - | 64.0 | 53.0 | 80.0 | 88.0 | 47.0 | 6.0 | 89.3 | [48] | |
| Liometopum apiculatumd | 58.0 | 29 | 50.0 | 42.0 | - | - | - | - | - | 18.0 | 14.0 | 60.0 | 49.0 | 76.0 | 39.0 | 68.0 | 8.0 | 88.0 | [48] | |
| Macrotermes bellicosus | 54.2 | 51.4 | 69.4 | 27.5 | - | - | - | - | - | 7.5 | 18.7 | 73.3 | 51.1 | 78.3 | 43.8 | 30.2 | 14.3 | 97.3 | [46] | |
| Bombyx mori (larvae) | 47.3 | 25.8 | 41.9 | - | 31.2 | 36.6 | 100.0 | 34.4 | 60.2 | 45.2 | 14.0 | 8.6 | 40.9 | 32.3 | 52.7 | 29.0 | 31.2 | 7.5 | 64.8 | [49] |
| Acheta domesticus (adults) | 53.7 | 23.4 | 61.0 | - | 36.1 | 49.8 | 104.9 | 56.1 | 50.7 | 87.8 | 14.6 | 8.3 | 52.2 | 45.9 | 100.0 | 31.7 | 48.8 | 6.3 | 78.2 | [49] |
| Boopedon flaviventrish | 55.0 | 24.0 | - | 44.0 | - | - | - | - | - | 18.0 | - | 57.0 | 47.0 | 88.0 | 41.0 | 74.0 | 6.0 | 83.1 | ||
| Fish (Clarias anguillaris) | 50.2 | 11.8 | 47.8 | 70.4 | 20.8 | 19.2 | 118 | 24.5 | 31.1 | 24.8 | 23.4 | 7.3 | 28.0 | 25.8 | 64.7 | 38.7 | 24.6 | - | - | [50] |
| Beef | 45 | 20 | 33 | 52 | 25 | 27 | 90 | 28 | 24 | 30 | 16 | 5.9 | 20 | 16 | 42 | 24 | 22 | - | - | [51] |
2.3. Fatty Acids Composition
| Common Name | Fat Content |
Saturate d Fatty Acids (SFA) | Monounsaturated Fatty Acids (MUFA) | Polyunsaturated Fatty Acids (PUFA) | Linoleic Acid (18:2) | Alfa Linolenic Acid (18:3) | Arachidonic Acid (18:4) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Winged termite | 44.82 | 35.05 | 52.77 | 12.18 | 10.75 | 1.43 | [55,56] | |
| White spotted flower beetle | 26.70 | 23.61 | 95.20 | 10.40 | 9.10 | 0.40 | 0.70 | [27,57] |
| Desert locust | 13.00 | 25.30 | 39.35 | 26.28 | 14.04 | 11.35 | [58] | |
| Dung beetle * | 13.50 | 733.46 | 85.65 | 1514.32 | - | 39.82 | 934.95 | [59,60] |
| Black soldier fly larvae | 35.00 | 36.20 | 28.70 | 35.00 | 13.00 | 1.70 | - | [61,62,63] |
| Sugarcane termite | 46.00 | 32.17 | 56.10 | 11.73 | 11.54 | 0.20 | - | [56] |
| Tropical house cricket | 20.00 | 33.74 | 34.33 | 31.91 | 29.78 | 2.13 | - | [58] |
| Guizhou black ant |
15.20 | 23.90 | 72.40 | 3.70 | 2.10 | 1.00 | 0.20 | [64] |
2.4. Micronutrient and Vitamin Composition Variability
3. Production of Protein, Protein Hydrolysate, and Peptides
4. Hydrolysis of Insect Proteins
5. Bioactive Peptides
| Insect Source | Enzyme Used | Identified Peptides | Bioactivity | Reference |
|---|---|---|---|---|
| Silkworm/domestic silk moth (Bombyx mori) | Pepsin, trypsin, R-chymotrypsin | simulated the human gastrointestinal hydrolysate | ACE inhibitory effects, with 100% activity in hydrolysates | [85] |
| Cotton leafworm (Spodoptera littoralis) | Pepsin, trypsin and α-chymotrypsin | Ala-Val-Phe | In vitro ACE inhibitory activity (IC50: 2123 μM) | [95] |
| Mealworm larva (Tenebrio molitor) | Alcalase | Tyr–Ala–Asn | ACE inhibitory activity (IC50: 0.017 mg/mL) | [89] |
| Silkworm pupae (Bombyx mori) | Alcalase, Prolyve, Flavourzyme, Brewers Clarex | SWFVTPF, NDVLFF | Antioxidant activity (ROS reduction, superoxide dismutase (SOD) expression and glutathione (GSH) production activity) | [90] |
| Tropical house crickets (Gryllodes sigillatus) | Alcalase-generated protein hydrolysates | YKPRP, PHGAP, VGPPQ | Anti-hypertensive, anti-glycemic, anti-inflammatory activities | [91] |
| Black soldier fly (Hermetia Illucens) | -------------------------- | Hill-Cec1, Hill-Cec10 | Antimicrobial activity against various pathogens | [96] |
| Silkworm pupae (Bombyx mori) | Acidic protease, Neutral protease | FKGPACA, SVLGTGC | Antioxidant activity, ABTS radical scavenging | [97] |
| Crickets (Gryllus bimaculatus) | Alcalase | TEAPLNPK, EVGA, KLL, TGNLPGAAHPLLL, AHLLT, LSPLYE, AGVL, VAAV, VAGL, QLL | Antioxidant activity | [98] |
| White-spotted flower chafer larva (Protaetia brevitarsis) | Flavourzyme | Ser-Tyr, Pro-Phe, Tyr-Pro-Tyr, Trp-Ile | ACE inhibitory activity, NO production in cells | [99] |
| Brown seaweed (Laminaria digitate) | Hydrolysates, fermentation generated peptides or generated from high-pressure processing | YIGNNPAKGGLF, IGNNPAKGGLF, and others (130 in total) | ACE-1 inhibitory activity, Potential DPP-IV inhibition | [100] |
6. Bioactive Peptides from Insect Proteins: Functional Potential and Applications
6.1. Protein Solubility
Zielińska, Karaś, and Baraniak [69] studied the functional properties of proteins isolated from three species of edible insects including the tropical house cricket, desert locust, and mealworm, and evaluated the solubility of the isolated proteins. Their research found that the proteins exhibited their lowest solubility at a pH level of approximately 5 across all three protein samples, while the highest solubility was achieved at around pH 11. A high protein solubility was also shown within the pH range of 2 to 4. Similar results have been reported for larvae of an edible insect, the black soldier fly, in which the lowest solubility was shown at pH values of 4 to 5. However, the solubility increased beyond this range [78]. Mexican fruit fly protein also showed the minimum protein solubility at pH 5 whereas its maximum solubility was at pH 10 [66].
6.2. Edible Insect Proteins as Emulsifiers
Zielińska, Karaś, and Baraniak [69] compared the emulsifying properties of three insect species in which the highest value was noted in the tropical house cricket protein preparation (72.62%) with an emulsion stability of 38.3%. Similarly, Omotoso [103] reported an emulsion activity of 75% for silkworms but with a lower emulsion stability (23%). The emulsion activities for the whole insects in the Zielińska, Baraniak, Karaś, Rybczyńska, and Jakubczyk [58] study were found to have consistent values ranging from 62% to 69.17%. Moreover, pretreatment with pulsed-field electricity (PFE) could improve the emulsifying capacity (EC) of proteins extracted from the house cricket flour. The highest intense PFE increased the emulsifying capacity to 74.7%, while the lowest increase in the EC was 22.1%.
6.3. Foaming Properties of Insect Proteins
Egg-white proteins are the most widely utilized proteins in spray applications. Furthermore, milk proteins such as whey proteins and caseins or soy proteins are also utilized for spray utilization. The characteristics of foam inherent to these proteins have been investigated, especially within the most popular edible insect proteins. These protein sources exhibit potential for integration into food formulations for foaming purposes under specific pH and ionic strength conditions [101]. The foaming activity and stability are different among various insects, even when subjected to a similar processing approach. One of the main factors affecting the foaming properties is the amino acid composition of insect proteins, as shown in Kim, et al. [107]. In that study, the salt-soluble protein fraction of mealworms revealed significantly higher foamability than water-soluble fractions. Having a polar water-soluble group (amphiphilicity) is a critical parameter in foaming properties. No foam activity was observed in the African migratory locust flours at a pH of less than 3, while a significant foam formation of around 200% was shown at pH 5 [108].
6.4. Gelling Properties of Insect Proteins
6.5. Water-Holding Capacity of Insect Proteins
All conditions associated with the protein matrix’s capacity to retain the maximum amount of water per gram of the sample material, even when subjected to the force of gravity, are called the water-holding capacity (WHC). Water-holding capacity (WHC), water-binding capacity (WBC), and water absorption capacity (WAC) are all the same terms used to elucidate how a protein is able to absorb water. Despite potential variations in measurement methodologies, these three terminologies are commonly employed interchangeably. This functional characteristic is indeed related to the gelation and gelling properties of proteins. Furthermore, the water-binding capacity is enhanced through thermal denaturation. This feature is also linked to a better texture and wetness that is of considerable relevance in food formulation. Certain insects like mealworm and African palm weevil (Rhynchophorus phoenicis) have greater WHC values than pulse protein flours and are similar to concentrated soy and milk protein levels. These interesting results show that specific concentrated insect protein or flour may be included as functional agents in food formulation [101].
6.6. Oil Absorption Capacity of Insect Proteins
7. Edible Insects and Their Proteins in Meat Analogs and Cereal Products
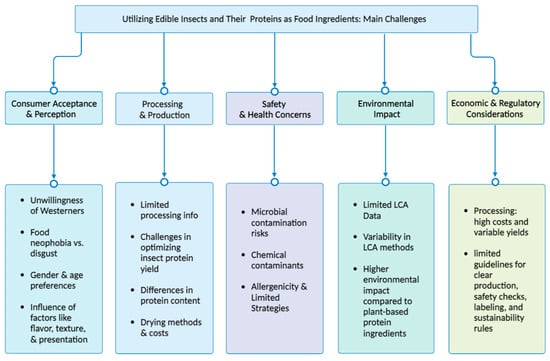
8. Challenges, Food Safety, and Considerations in Utilizing Edible Insects and Their Proteins as Food Ingredients
9. Halal and Kosher Considerations for Insect-Based Food Products
This entry is adapted from the peer-reviewed paper 10.3390/foods12234243
