Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Ischemic stroke is a leading cause of death and disability in the world. At present, reperfusion therapy and neuroprotective therapy, as guidelines for identifying effective and adjuvant treatment methods, are limited by treatment time windows, drug bioavailability, and side effects. Nanomaterial-based drug delivery systems have the characteristics of extending half-life, increasing bioavailability, targeting drug delivery, controllable drug release, and low toxicity, thus being used in the treatment of ischemic stroke to increase the therapeutic effects of drugs.
- ischemic stroke
- nanomaterials
- nanoparticles
- drug delivery
1. Introduction
Ischemic stroke is a leading cause of death and disability in the world [1]. To date, the effective therapy for ischemic stroke is to restore cerebral blood flow via intravenous thrombolysis or mechanical thrombectomy [2]; however, the therapy is limited to narrow time widows [2], and even timely restoration of cerebral blood flow may not prevent long-term neurological deficits, because of secondary neuronal damage caused by reperfusion [3]. These damaging pathological processes (i.e., inflammation, oxidative stress, and excitotoxicity) persist in the brain during the acute stage and even in the sequelae stage [3]. Thus, targeting these pathological processes has great therapeutic prospects for neuroprotection to improve neuronal survival and outcomes after ischemic stroke.
At present, numerous neuroprotective drugs have been developed, but almost all failed in clinical translation [4]. There are some important explanations for this translational failure. On the one hand, it lies in the insufficient concentration of drugs that reach the ischemic area because of the limitations of the blood–brain barrier (BBB) [5]. Although a damaged BBB causes an increase in drug penetration during the acute stage of ischemic stroke, it will be closed again long after ischemic stroke [6]. On the other hand, faced with multiple pathological processes, i.e., inflammation, oxidative stress, and excitotoxicity, a single treatment option cannot salvage the ischemic penumbra. Thus, some researchers have developed drug conjugates with synergistic neuroprotection effects for targeting different pathological processes to reduce the damage of neurons to minimize the ischemic area [7,8]. Another important reason is that it is difficult for drugs to target specific damaged cells. There are many different types of cells, i.e., microglia, astrocytes, and neutrophils, involved in pathological processes, and targeting drug delivery to damaged cells may prevent the side effects of drugs [3]. Therefore, novel technologies that can target drug delivery to an intended area or cells, selectively release drugs, and increase the bioavailability of drugs are promising for the treatment of ischemic stroke.
Nanomaterial-based drug delivery systems have gradually been attracting attention in recent years because of their specific characteristics of targeting drug delivery, controllable drug release, biocompatibility, biodegradability, and low toxicity [9]. Recently, many studies have confirmed that nanomaterials can carry drug targets and cross the BBB, after which they can then conduct secondary targeted drug delivery, and then the drug is released to the damaged tissue and cells in ischemic stroke [10]. Therefore, nanomaterials have great prospects in optimizing the treatment of ischemic stroke. There are several reviews that have reviewed drug delivery systems based on thrombus, the brain–blood barrier (BBB), and ischemic brain parenchyma in ischemic stroke, as well as the different approaches for central nervous system (CNS) drug delivery [11,12,13,14].
2. The Pathogenesis of Ischemic Stroke
Ischemic stroke is a neurological disease characterized by cerebral vascular occlusion. Oxygen depletion, neuroinflammation, and oxidative stress are the main reasons for poor prognoses of ischemic stroke [3,15,16]. Following the interruption of cerebral blood flow, neurons in brain areas dominated by blocked blood vessels will experience hypoxia [15]. Oxygen depletion leads to the irreversible necrosis of neurons, after which a cerebral infarction core is formed [15]. In addition to neuronal necrosis, the BBB is disrupted in ischemic stroke [6]. The BBB is mainly composed of vascular endothelial cells, tight junction proteins (including claudin-5, occludin, and ZO-1), pericytes, and astrocytes [10]. In normal conditions, the BBB could prevent macromolecular substances and peripheral blood cells from entering the brain [10]. Within 120 h of reperfusion after focal cerebral ischemia, BBB permeability is significantly increased, and two peaks appear at 3 h and 72 h [17,18], resulting in leukocytes (i.e., neutrophils and monocytes) in peripheral blood and macromolecular substances entering the ischemic brain, thus further aggravating brain damage [6,19]. The infiltration and accumulation of leukocytes in cerebral ischemic areas depends on the function of adherent proteins expressed on neutrophils, such as integrin αMβ2, CD44, CD11b, macrophage-1 antigen (Mac-1), and lymphocyte function-associated antigen 1 (LFA-1) [20]. During ischemia and reperfusion, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM)-1, and P-selectin are overexpressed on stressed vascular endothelial cells [6,21]. These molecules can drive leukocytes in peripheral blood to adhere to inflammatory vascular endothelial cells and enter cerebral ischemic areas via membrane-adherent proteins expressed on leukocytes [6,21]. The activation of resident microglia in the brain and the infiltration of leukocytes into the brain result in neuroinflammatory responses that further exacerbate neuronal death [6,22]. In fact, M1-microglia- and leukocyte-mediated neuroinflammatory responses will persist for 1 month after ischemic stroke, which is one of the main reasons for the poor prognoses of ischemic stroke [6,23]. Furthermore, the excessive production of reactive oxygen species (ROS) (i.e., H2O2, O2−, ·OH, and HOCI) after cerebral ischemia onset results in oxidative stress responses, which is also the leading reason for aggravated neuronal death [16,24,25]. Therefore, ensuring the supply of oxygen to the brain and inhibiting neuroinflammatory as well as oxidative stress responses are key to protecting neurons from death and saving ischemic penumbra in ischemic stroke. The abnormally expressed proteins and cells mentioned above may become therapeutic targets for ischemic stroke.
3. Nanomaterials Applied in the Treatment of Ischemic Stroke
Reperfusion through intravenous thrombolysis or mechanical thrombectomy is an effective method for treating ischemic stroke [2,26]; however, there is a strict time window limit [2,26]. Even if patients receive reperfusion treatment in time, it is difficult to avoid reperfusion injury after ischemic stroke [3,27]. Reperfusion injury is mainly caused by infiltrated leukocytes and overproduced oxygen radicals after a sudden increase in oxygen, thus resulting in no reflow of blood after the reperfusion of ischemic stroke, which can even worsen neuronal death [27]. Therefore, numerous neuroprotective drugs have been developed to prevent neuronal damage after reperfusion and extend the therapeutic time window to further improve ischemic stroke prognoses [28]. These neuroprotective drugs, such as those with anti-inflammatory and antioxidant stress effects, have been widely studied in animal and cell experiments, and their therapeutic effects have been confirmed in ischemic stroke models [28]; however, these neuroprotective drugs failed in clinical conversion [29,30]. The main reasons for their failure are attributed to an insufficient concentration of drugs in the ischemic brain and the quick elimination of drugs in peripheral blood. Their low absorption, poor bioavailability, poor targeting, and short half-lives result in insufficient concentrations of drugs in cerebral ischemic areas. In view of these limitations, nanomaterial-based drug delivery systems are widely applied in the treatment of ischemic stroke due to their specific properties of targeted drug delivery, controllable drug release, biocompatibility, biodegradability, and low toxicity [10,11,31]. Furthermore, nanoparticles could integrate multiple therapy approaches for ischemic stroke [7,32,33]; for example, Yuan fabricated a nanoparticle with the dual function of antioxidative and anti-inflammatory activities to target the treatment of ischemic stroke [7]. S. elongatus, a type of cyanobacteria, is encapsulated in nanoparticles and delivered to cerebral ischemic areas to generate oxygen and absorb carbon dioxide, thus rescuing neurons in the ischemic penumbra area [33]. In addition to delivering drugs to cerebral ischemic areas, leukocyte membrane-derived nanovesicles could inhibit the accumulation of neutrophils and monocytes in ischemic brain areas by consuming the binding sites with neutrophils or monocytes [34]. Therefore, it is essential to understand nanocarriers, the targeting mechanisms of nanoparticles, and drug release mechanisms to further improve the use of nanomaterials in the treatment of ischemic stroke. The possible function and mechanism of nanomaterial-based drug delivery systems in ischemic stroke are showed in Figure 1.
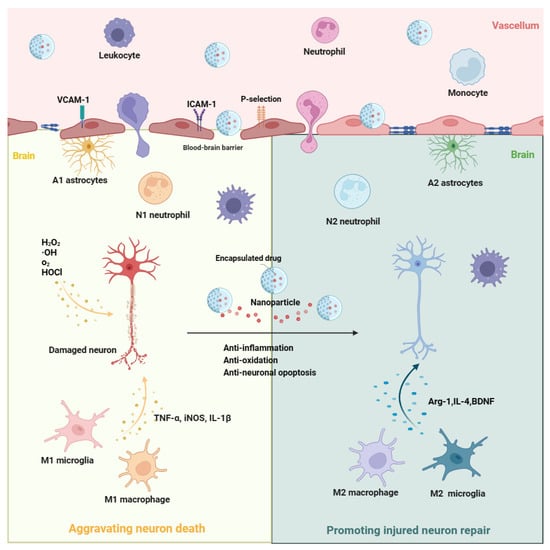
Figure 1. The possible function and mechanism of nanomaterial-based drug delivery systems in ischemic stroke. After ischemic stroke, M1 microglia and A1 astrocyte are rapidly activated in ischemic brain areas. In addition, leukocytes (including neutrophils and monocytes) in peripheral blood are recruited into the ischemic brain area. Among them, M1 microglia, A1 astrocyte, N1 neutrophils, and M1 macrophages would aggravate the neuron death through the overproduction of inflammatory molecules (i.e., TNF-α, iNOS, and IL-1β) and ROS (i.e., H2O2, O2−, ·OH, and HOCI). Based on the nanomaterial-based drug delivery systems, these harmful cell phenotypes may be shifted towards beneficial phenotypes (M2 microglia, A2 astrocyte, N2 neutrophils, and M2 macrophages) to exert the function of anti-inflammation, anti-oxidation, and anti-neuronal apoptosis, thus promoting injured neuron repair in ischemic stroke through the release of drugs or genes that are encapsulated in the nanoparticles. ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; H2O2, hydrogen peroxide; •OH, hydroxyl radicals; O2, oxygen; HOCl, hypochlorous acid; TNFα, tumor necrosis factor α; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β; Arg-1, arginase-1; IL-4, interleukin-4; BDNF, brain-derived neurotrophic factor.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15122669
This entry is offline, you can click here to edit this entry!
