Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
Organic light-emitting diodes (OLEDs) have emerged as a promising technology for various applications owing to their advantages, including low-cost fabrication, flexibility, and compatibility. However, a limited lifetime hinders the practical application of OLEDs in electronic devices. OLEDs are prone to degradation effects during operation, resulting in a decrease in device lifetime and performance.
- analytical techniques
- degradation
- failure modes
- organic light-emitting diodes
1. Introduction
Organic light-emitting diodes (OLEDs) are poised to become the leading technology in high-quality flat-panel display [1] and solid-state lighting. Their disruptive features, including energy efficiency [2], wide viewing angles, fast response times, high contrast, and exceptional color purity (Figure 1a), make them ideal candidates for various applications (a few of them are shown in Figure 1b). In contrast, traditional LEDs require complex processing to achieve comparable light quality, and achieving uniform dispersion into a near-plane light source remains a challenge for them. A clear comparison of LEDs and OLEDs is shown in Figure 1c.
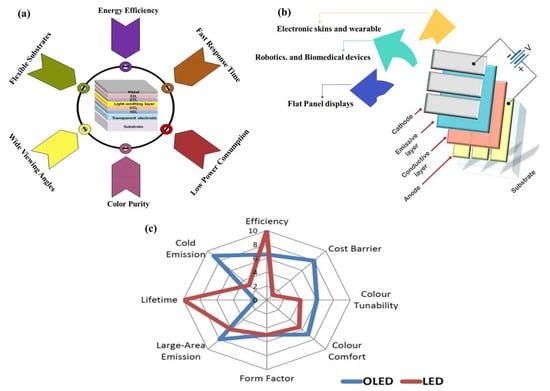
Figure 1. (a) Advantages of OLED. (b) Applications of OLED. (c) Comparison of OLED and LED. Reprinted from “The Global Information Hub for Lighting Technologies and Design”.
Moreover, OLED technology offers unique advantages in flexible and stretchable substrates [3], enabling applications in wearable electronics [4], biomedical devices [5], electronic skins [6], and robotics [7]. OLEDs’ thinness, flexibility, and durability allow them to withstand harsh mechanical conditions, such as bending and twisting. Additionally, OLEDs offer the ability to adjust color and color temperature over a wide range, allowing for the replication of natural light styles. While OLEDs excel in various applications, their use in general lighting presents cost challenges. The manufacturing costs of components increase proportionately when larger OLED lighting panels are required for uniform illumination in spaces like offices or reading areas. Due to their importance in industry and academia, OLEDs are receiving much focus in research with a large number of research articles published every year on the topic, shown in Figure 2 and Figure 3.
While OLED researchers and market analysts are optimistic about OLED technology disrupting conventional lighting and capturing a significant market share, it is crucial to streamline market-related content. The Global OLED Display Market, valued at 43,066 million USD in 2022, is projected to reach 11,709,730 million USD by 2028, with a CAGR of 18.14%. This growth is driven by the rising adoption of OLED technology, advancements in display technology, and increasing consumer demand for high-quality displays.
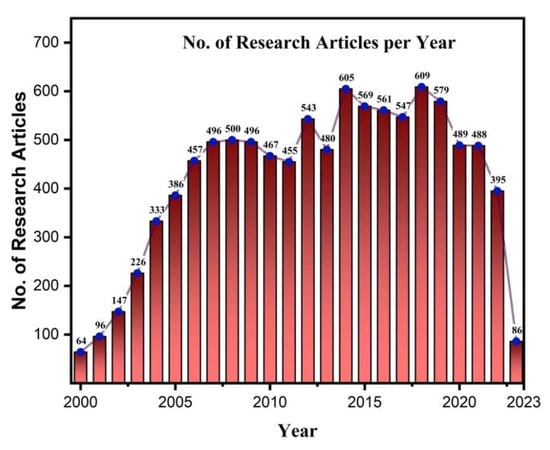
Figure 2. No. of research articles published per year till now on OLED (Web of Science).
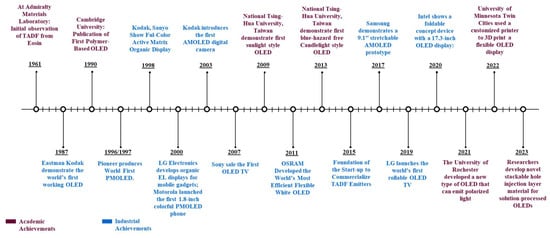
Figure 3. Academic and industrial achievements of OLED.
Researchers are exploring innovative strategies to enhance efficiency and mitigate degradation in organic light-emitting diodes (OLEDs) [8]. A notable approach involves the utilization of thermally activated delayed fluorescence (TADF) materials, capable of converting less efficient triplet excitons into more efficient singlet excitons. This holds the potential for significant improvements in OLED efficiency [9]. Another avenue is the adoption of triplet–triplet annihilation (TTA), a process wherein two triplet excitons combine to form a singlet exciton, contributing further to enhanced OLED efficiency. Despite some limitations of TADF OLEDs, such as broad emission spectra and longer lifetimes, the technique of hyperfluorescence proves effective in overcoming these constraints [10]. Hyperfluorescence entails employing TADF emitters as assistant hosts for fluorescent end-emitters, resulting in OLEDs with up to 100% internal quantum efficiency (IQE) [11]. The introduction of TED-TADF, a novel OLED technology amalgamating the advantages of TADF and TTA, demonstrates the efficient harnessing of triplet excitons and their conversion into singlet excitons, leading to heightened device efficiency and prolonged operational life, particularly in deep-blue OLEDs [12,13]. Overall, numerous promising approaches to enhancing OLED efficiency, including TADF, TTA, and TED-TADF, are showing significant potential, with ongoing research aimed at refining techniques for further improvements.
Despite the numerous advantages, one significant challenge that OLEDs face is degradation during operation, which can lead to a decrease in device lifetime and performance. OLED degradation mechanisms can occur internally or be influenced by external factors. Internal degradation processes involve reactions within the OLED materials, such as the degradation of organic layers or the formation of non-radiative recombination sites. One of the most common intrinsic degradation mechanisms in OLEDs is the formation of excitons (electron–hole pairs) in the organic layers. These excitons can generate reactive species, such as free radicals and triplet excitons, which react with organic materials, forming non-emissive species and reducing device efficiency [14,15]. Additionally, the diffusion of organic molecules from the emissive layer into adjacent layers or the substrate can result in the formation of non-emissive species or chemical reactions with other materials in the device. Metal ions diffusing from the cathode into the organic layers can also contribute to reduced efficiency [16]. External factors, such as moisture, oxygen, and UV radiation exposure, can also contribute to OLED degradation. Moisture and oxygen can react with organic materials and affect their properties, impacting efficiency and lifetime. Light exposure can generate reactive species like singlet oxygen, which can also lead to the formation of non-emissive species [17]. OLED degradation can also occur through various factors, including chemical, electrical, thermal, and optical degradation. Chemical degradation is caused by exposure to environmental factors like oxygen and moisture, leading to reduced efficiency [18]. Electrical degradation occurs due to charge buildup in the organic layers, resulting in chemical reactions and device degradation [19]. Thermal degradation occurs when OLEDs are exposed to high temperatures, degrading organic materials and decreasing efficiency [20,21,22]. Optical degradation occurs when OLEDs are exposed to light, causing degradation of the organic materials [23].
2. The Lifetime
OLEDs may undergo internal decay through various mechanisms like chemical reactions, morphological changes, and physical changes like charge accumulation. These mechanisms can impact the device’s color, luminance, current, and voltage properties, which may eventually reduce the device’s efficiency and lifetime. The luminance behavior of OLEDs over time typically assessed at a constant physical environment such as temperature, current density, voltage, or humidity, is the most critical factor in determining the device’s lifetime. The device lifetime is usually expressed as T50 or T1/2, the duration for the luminance to dim to half its initial brightness at a constant current density [31]. More complex metrics such as T70 and T97 measure how long it takes for the luminance to drop to 70% and 97% of its initial value, respectively. The T97 metric is significant for the display industry as it measures the threshold at which humans can perceive differences in brightness between adjacent display elements. Although some researchers have assessed device lifetime using constant voltage sources, this can cause luminance drops to occur more quickly as the device’s impedance rises with time, decreasing current density.
Interestingly, the luminance of OLEDs often varies directly with the current density across a wide range. This suggests that as the current density delivered to the OLED increases, its lifetime typically decreases, according to the well-known empirical scaling law Ln0⸱T1/2=CL0n⸱T1/2=C [31,32,33]. In fact, researchers have found they can predict the lifetime of very stable OLEDs with reasonable accuracy by studying the relationship between a device’s initial luminance at time zero (L0) and its lifetime (T1/2), using a constant factor (C), as described in studies by Meerheim et al. and Jarikov et al. [34,35]. The rate of degradation of a device over time can be influenced by the acceleration factor (n), which is specific to the materials and design of the device under study. However, accurately predicting the lifetime of very stable organic devices can be challenging due to the potential for inaccuracies [33,34]. In some cases, it can be difficult to predict an OLED’s actual lifetime since various factors can cause degradation. Researchers may observe an initial sharp decline in luminance followed by a more gradual, long-term decline. To account for these complex patterns, researchers use a combination of different exponential decay functions, such as LtL0/=ae−αt+be−βtLtL0=ae−�t+be−�t. This equation includes several fitting parameters, including a, b, α, and β, which can be used to predict the OLED’s lifetime more precisely [33]. The alternative method for predicting the lifetime of OLEDs is through the stretched exponential decay (SED) function, which is represented by, LtL0=exp[−(tτ)β]LtL0=exp[−t��] [33,34,36]. The degradation of OLEDs is linked to the formation of defects within the device, which can function as luminescent quenchers, nonradiative recombination centers, or deep charge traps based on their energy levels [37,38]. It has been found that at 50% luminance loss in a particular system, the areal density of fixed charges linked with these defects is around 6 × 1011 cm−2 (or 10−7 C/cm2) in size [39]. Giebink et al. investigated electroluminescence loss utilizing a mathematical model with various arbitrary factors, which showed that a defect density of 1018 cm−3 (about 0.1% of the molecular density) might cause a loss of more than 50% of the device luminance [38]. However, it is crucial to remember that these equations might underestimate or overestimate the lifetime if inadequate data were utilized for fitting [34,40]. The SED function has two fitting parameters, τ and β, representing the decay constant and stretching factor, respectively, but neither holds any specific physical significance. It has been empirically found that β varies for different material configurations and stack designs but remains the same for various current densities of identical OLED stacks.
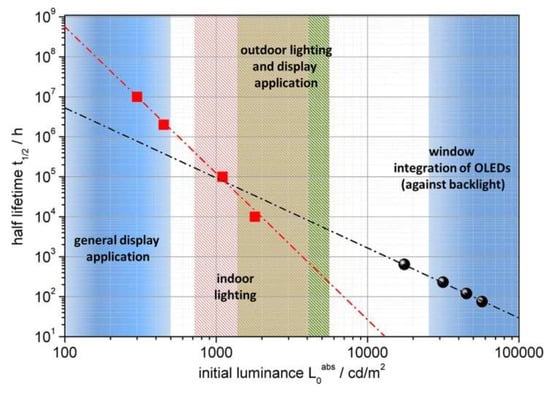
The way lifetime data are reported and compared has changed over the last few years, in line with the criteria for device application by industrial standards. An overview of relevant OLED brightness, needed for several applications is shown in Figure 4. Initially, device lifetime was commonly reported at an expected display brightness of 100 cd/m2, but, nowadays, the majority of lifetime data are published as luminance, which is 1 order of magnitude higher, i.e., 1000 cd/m2, suitable for both simple lighting applications and demanding display applications. It is well-known that indoor displays usually have an overall brightness ranging from 100 to 300 cd/m2 [41]. To attain this range of brightness, individual pixels on the device must emit light at levels between 200 and 600 cd/m2, depending on the pixel’s size, color, and device architecture [32,42]. In 2001, Howard and Orache from IBM and Chwang et al. from UDC predicted that a display would need to last for over 10,000 h at 140 cd/m2, assuming continuous operation and integration into devices such as entertainment headsets [36,43]. The incorporation of filters such as polarizers in displays also increases the device lifetime requirements. According to the same IBM study, even modest outdoor displays may require brightness as high as 3000 cd/m2. This requirement extends to the industry and governmental bodies, as evidenced by the German Federal Ministry of Education and Research project to achieve a lifetime of 30,000 h for white OLEDs at 1000 cd/m2.
This entry is adapted from the peer-reviewed paper 10.3390/nano13233020
This entry is offline, you can click here to edit this entry!