Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Endocrinology & Metabolism
Diabetes mellitus, a chronic metabolic disorder characterized by hyperglycemia, presents a formidable global health challenge with its associated complications. Adiponectin, an adipocyte-derived hormone, has emerged as a significant player in glucose metabolism and insulin sensitivity. Beyond its metabolic effects, adiponectin exerts anti-inflammatory, anti-oxidative, and vasoprotective properties, making it an appealing therapeutic target for mitigating diabetic complications.
- adiponectin
- AdipoRs
- diabetes
- pancreatic islets
1. Adiponectin
Adiponectin, alternatively referred to as AdipoQ, APM1, or ACRP30, is a single-chain adipokine composed of 244 amino acids, possessing a molecular weight of around 26 kilodaltons (kDa) secreted by white adipose tissue. The adiponectin protein is encoded by the AdipoQ gene located on the chromosome locus 3q27. Adiponectin consists of several distinct structural components. It includes an NH2-terminal hyper-variable region, a collagenous domain consisting of 22 Gly-XY repeats, and a COOH-terminal C1q-like globular domain. When secreted into the bloodstream, adiponectin forms three oligomeric complexes: a trimer, a hexamer, and a high molecular weight multimer [1]. Adiponectin primarily binds to seven transmembrane receptors known as AdipoR1 and AdipoR2 to regulate a range of physiological functions including whole-body energy balance, inflammatory responses, insulin sensitivity, and the process of fat metabolism [2]. Unlike traditional G-protein coupled receptors, these receptors possess a cytoplasmic NH2 terminus and an extracellular COOH terminal domain. AdipoR1 is most abundantly expressed in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver [3]. In humans and mice, AdipoR1 is situated on chromosome 1p36.13-q41, while AdipoR2 is found on chromosome 12p13.31 and 6 F1. The molecular structure of both forms of the receptor exhibits significant homology, featuring an internal N-terminus and an external C-terminus [4]. AdipoR1 and AdipoR2 (Figure 1) are adiponectin receptors that stimulate AMP-activated kinase (AMPK) and PPAR activity, regulating glucose and lipid metabolism. Adiponectin-induced complete AMPK activation requires both Ca2+/CaMKK and AMP/LKB1 [5].
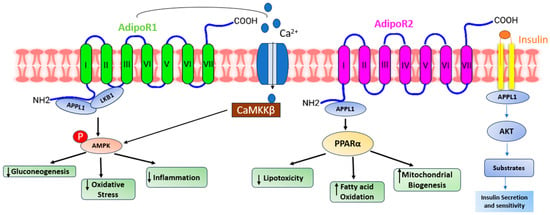
Figure 1. A presentation demonstrating the diverse pathways through which adiponectin receptors exert their functions. Adiponectin engages with its receptors to initiate various signaling pathways. AdipoR1 enhances calcium influx, leading to the activation of Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) and subsequent downstream kinases. AdipoR1- and R2-dependent signaling are mediated by adaptor protein phosphotyrosine interaction (APPL) 1, which allows LKB1 to translocate from the nucleus to the cytoplasm and activate AMPK, ceramidase activity, and peroxisome proliferator-activated receptor-alpha (PPAR-α). Activation of AMPK reduces gluconeogenesis, oxidative stress, and inflammation. On the other hand, activation of PPAR-α reduces lipotoxicity and inflammation and increases fatty acid oxidation. All these effects ultimately improve glycemic status.
Adiponectin stimulates glucose uptake and fatty acid oxidation in skeletal muscle after binding to AdipoR1, which is mediated by the recruitment of the adaptor protein with the pleckstrin homology domain, phosphotyrosine domain, and leucine zipper domain (APPL). APPL binding to the intracellular domain of AdipoR1 activates Rab5, a small GTPase that enhances GLUT4 membrane translocation and glucose absorption in muscle. APPL also binds to PI3 kinase and Akt, showing that adiponectin can boost insulin signaling as well [6]. The interaction between APPL and AdipoR1 activates AMP-activated protein kinase (AMPK), which inhibits acetyl-CoA carboxylase (ACC) and promotes fatty acid oxidation—adipoR-mediated activation of AMPK leads to higher fatty acid oxidation and decreased obesity. AMPK activation increases glucose absorption and lactate generation in muscle while suppressing gluconeogenesis. Together, the adiponectin signaling pathways underscore the relevance of adiponectin in glucose and lipid metabolism (Figure 2) [7].
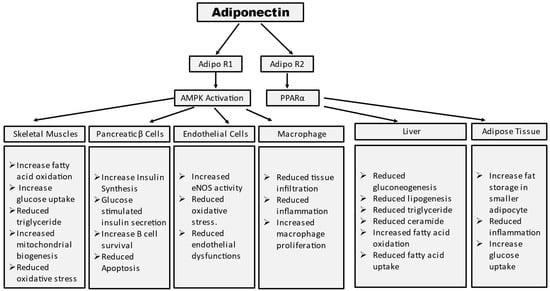
Figure 2. Summary of tissue-specific functions of adiponectin. Mechanism of adiponectin actions in prevention of insulin resistance and diabetes.
2. Adiponectin Pathway Regulation
2.1. Genetic Factors
Adiponectin is mostly generated in white adipose tissue by mature adipocytes. Originally assumed to be expressed solely by adipose tissue, it is now widely documented that adiponectin is generated and released by a variety of cell types, including skeletal and cardiac muscles [8][9]. The normal range of adiponectin in human plasma is 2–20 μg/mL. Adiponectin levels are also regulated by genetic variations in the ADIPOQ gene, which encodes adiponectin. Some genetic polymorphisms are linked to increased or decreased adiponectin production [10]. The adiponectin gene, situated on chromosome 3q27, corresponds to a susceptibility locus for diabetes. Various cross-sectional studies have demonstrated an association between single-nucleotide polymorphisms (SNPs) within the adiponectin gene (ADIPOQ) and diabetes. These SNPs within ADIPOQ play a crucial role in evaluating the link between common genetic variants, adiponectin levels, and the risk of diabetes.
2.2. Insulin Sensitivity
Insulin sensitivity is one of the most important determinants of adiponectin levels. Higher levels of adiponectin correspond to greater insulin sensitivity. On the other hand, insulin sensitivity is reduced in several diseases such as diabetes and obesity and the adiponectin level also decreases [11]. A team of researchers from the Karolinska Institutet in Stockholm,
2.3. Inflammatory State
The levels of adiponectin in the bloodstream decline following an elevation in proinflammatory cytokines like TNF-α and IL-6, along with endothelial reticulum stress and adipocyte hypertrophy. This phenomenon is associated with conditions linked to expanded adipose tissue, including obesity, type 2 diabetes mellitus (T2DM), cardiovascular disease, and metabolic syndrome [12].
2.4. Adipose Tissue Distribution
The distribution of adipose tissue affects the levels of adiponectin. Subcutaneous adipose tissue is associated with higher adiponectin levels compared to visceral fat [13].
2.5. Diet and Nutritional Factors
Several dietary components such as omega-3 fatty acids and polyphenols are regulated by the production of adiponectin [14]. A group of researchers from Universidade de São Paulo, São Paulo, Brazil observed in a double-blind, placebo-controlled, 2-month clinical trial with 80 individuals that supplementation of ω-3 fatty acid showed an increase in serum adiponectin [15].
2.6. Physical Exercise
Routine physical activities, particularly aerobic exercises like jogging or cycling, along with resistance training, can elevate adiponectin levels. Exercise also improves insulin sensitivity, which is associated with adiponectin secretion [16].
2.7. Hormonal Regulation
Leptin, another hormone secreted by adipose tissue, can affect adiponectin levels by opposing effects on metabolic regulation [17]. Insulin can stimulate adiponectin production and secretion. Improved insulin sensitivity, as seen with weight loss and exercise, can lead to increased adiponectin levels [18].
2.8. Adiponectin Receptors
Adiponectin shows its effects after binding to specific receptors, AdipoR1 and AdipoR2. Various tissues, including skeletal muscle, liver, and the cardiovascular system show expression of these receptors, which can affect the secretion of adiponectin [19]. Adiponectin receptors are expressed in skeletal muscle. When adiponectin binds to these receptors, it increases insulin sensitivity, which leads to improved glucose uptake by muscle cells and ultimately regulates blood glucose levels [20]. Adiponectin receptors are also expressed in the liver, which suppresses the production of excess glucose.
2.9. Aging
As individuals age, there is a decrease in the activity of brown adipose tissue, a decline in sex hormone levels, and an expansion of abdominal adipose tissue. This is accompanied by a shift of lipids from the subcutaneous fat compartment to the visceral fat compartment. This eventually results in reduced production of adiponectin [21].
2.10. Therapeutic Interventions
Certain medications and lifestyle interventions can affect adiponectin levels. Anti-diabetic drugs such as thiazolidinediones (TZDs) and metformin can increase adiponectin levels [22][23]. Thiazolidinediones (TZDs) elevate adiponectin levels, improving insulin sensitivity, enhancing AMPK activation, and reducing gluconeogenesis in the liver [24]. A meta-analysis showed that the administration of metformin led to a notable rise in serum adiponectin levels [25].
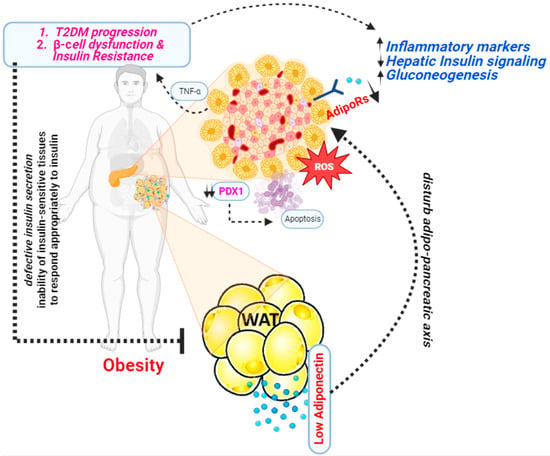
3. Adiponectin and Diabetes
In 1995, a group of researchers from the Whitehead Institute for Biomedical Research, Cambridge, Massachusetts first discovered adiponectin [26]. This was a significant discovery because at the time, adipose tissue was primarily viewed as a passive energy storage site. Studies revealed that adiponectin plays an important role in modulating insulin sensitivity. Higher levels of adiponectin have been linked to better insulin sensitivity, whereas lower levels have been linked to insulin resistance [27]. Several studies have shown a significant correlation between adiponectin levels and diabetes (Figure 3).

Figure 3. A presentation illustrating the mechanism through which adiponectin functions as an antidiabetic agent.
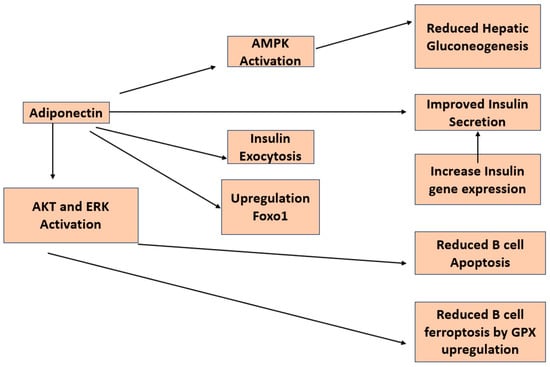
4. Insulin Resistance and Adiponectin
Insulin resistance has a hereditary component that is not fully understood and is frequently passed down through generations. Furthermore, obesity has a significant hereditary component that inevitably worsens insulin resistance. As a result, obesity and insulin resistance are often present for many years before additional alterations such as high blood pressure, dyslipidemia, T2DM, and cardiovascular disease develop [28][29]. It was discovered that, in both mice and humans, a reduction in adipose tissue leads to increased levels of circulating triglycerides and fatty acids. The presence of insulin resistance provides further substantiation for the pivotal role played by adipose tissue in governing systemic metabolism by lipid storage [30][31][32].
The first study to demonstrate that adiponectin actively affects insulin sensitivity was reported in 2001. A C-terminal globular adiponectin fragment can lower plasma glucose levels by boosting fatty acid oxidation in muscle [33][34][35]. Globular adiponectin appears to function in conjunction with AMP-activated protein kinase (AMPK) (and later by inhibiting acetyl-CoA carboxylase) and PPAR-α (peroxisome proliferator-activated receptor alpha) to create its metabolic action in the muscles. Ceramidase silencing can inhibit AMPK phosphorylation in C2C12 myotubes, indicating a function for sphingolipid metabolism with adiponectin signaling in this tissue. Adiponectin binding increases glucose uptake (through GLUT4 translocation) and non-oxidative glycolysis while decreasing intramyocellular triacylglycerol concentration and boosting fatty acid oxidation.
5. Apoptosis and Adiponectin
Liu et al. reported that, by stimulating the AdipoR1/AMP-activated protein kinase (AMPK) signal pathway, adiponectin decreased early apoptotic cells and prevented the mitochondrial apoptosis process in adipocyte culture. Furthermore, PPAR linked to the ATF2 promoter area and suppressed ATF2 transcription. ATF2 transcriptional suppression was associated with adiponectin’s ability to prevent apoptosis in adipocytes [36].
Treatment of beta-cell line INS-1 cells with a cytokine combination (IL-1b/IFN-c) or palmitic acid strongly promoted apoptosis, which could be greatly suppressed by gAPN via caspase-3 inhibition without altering NF-kB [37]. Lin et al. reported Adiponectin cotreatment partially reversed high glucose-induced INS-1 cell death, malfunction, and decrease in insulin gene expression, which was mediated at least in part by transiently activating the AMPK signaling system [38]. Adiponectin has also been identified to modulate several additional molecular pathways involved in apoptosis. This contains the Bcl-2 family of proteins, which are critical in managing the balance of pro-apoptotic (cell death-promoting) and anti-apoptotic (cell death-inhibiting) signals inside the cell. Adiponectin has the ability to modulate the expression and activity of Bcl-2, Bax, and Bak proteins [39].
Adiponectin may also have an effect on the tumor suppressor protein p53, which is important in starting apoptosis in response to cellular stress or injury. The action of adiponectin on p53 may contribute to its proapoptotic effects [40][41]. Adiponectin has also been demonstrated to affect the iNOS/ROS/RNS pathways. All molecules involved in cellular signaling and stress responses are iNOS (inducible nitric oxide synthase), ROS (reactive oxygen species), and RNS (reactive nitrogen species). The regulation of these pathways by adiponectin may contribute to its proapoptotic effects [42].
6. β-Cell Function and Adiponectin
There have been a number of studies looking at the direct impact of adiponectin on insulin secretion in β-cells (Figure 4). A group of researchers from the University of Tokyo reported that adiponectin enhances insulin release from isolated mouse islets by promoting the exocytosis of insulin granules, with no discernible impact on ATP production, KATP channels, membrane potential, calcium influx, or activation of AMPK [43]. An additional investigation demonstrated that adiponectin safeguards β-cells from apoptosis induced by prolonged serum deprivation and glucotoxicity. These outcomes are facilitated by the activation of both MEK-extracellular signal-regulated kinase (ERK) 1/2 and PI3K-Akt pathways [44]. James E P et al. reported that globular adiponectin induces a notable enhancement in cell viability, dependent on ERK1/2 signaling, along with a substantial rise in Pdx-1 expression in rat β-cell lines [45].

Figure 4. Diagram depicting the investigated pathways illustrating the impacts of adiponectin on pancreatic β-cells.
Adiponectin knockout mice exhibit compromised glucose tolerance, even in the presence of normal or lower-than-normal insulin levels [46]. Transgenic ob/ob mice expressing the globular domain of adiponectin demonstrate heightened insulin sensitivity and elevated insulin secretion in comparison to nontransgenic mice [47][48][49] In vivo experiments conducted in C57BL/6 mice revealed that intravenous administration of adiponectin leads to an augmentation in insulin secretion [43].
7. Oxidative Stress and Adiponectin
The production of reactive oxygen species (ROS) leads to oxidative stress, causing a range of cellular and molecular alterations, including dysfunction in mitochondria, which disrupt normal physiological processes in the body [50][51][52][53]. While oxidative pathways are crucial in mitochondrial-mediated processes, the exact molecular mechanisms responsible are still unclear. The compromised mitochondrial function is evident in insulin resistance across different cell types. Furthermore, ongoing research is unraveling the roles of the master antioxidant pathway involving nuclear factor erythroid 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), and antioxidant response elements (ARE) in elucidating various molecular pathways associated with diabetes. There are two contrasting theories regarding adiponectin and oxidative stress.
8. Anti-Inflammatory Functions of Adiponectin
Numerous metabolic strains that contribute to insulin resistance and T2DM also trigger the activation of inflammation- and stress-related enzymes, namely IκB kinase-β (IKKβ) and JUN N-terminal kinase (JNK). This implies that these kinases likely play pivotal roles in the development of these disorders [54]. Specifically, IKKβ initiates the activation of the transcription factor nuclear factor-κB (NF-κB), and obesity leads to heightened expression of NF-κB-regulated genes, such as pro-inflammatory cytokines, in both the liver and adipose tissue [54]. These cytokines, encompassing TNF, IL-6, and IL-1β, can potentially induce insulin resistance in the originating tissues like the liver and adipose tissue [55].
A number of experimental studies with genetic loss-of-function manipulations indicate that ablation of adiponectin contributes to diet-induced insulin resistance, increased vascular remodeling in response to injury, and severe cardiac damage under ischemic conditions [56]. A sequence of in vitro experiments has shown that adiponectin has the capacity to impede the production and effects of TNFα, which is a pivotal proinflammatory cytokine. This effect has been observed in different types of cells, including cardiac and vascular cells [57].
9. Conclusions
In conclusion, the significance of adiponectin in diabetes management cannot be overstated. Its pivotal role in regulating glucose metabolism and insulin sensitivity highlights its potential as a promising therapeutic target. As research in this sector advances, tapping the full potential of adiponectin may lead to novel and successful diabetes therapies. Further research, including clinical trials and in-depth molecular investigations, will be critical in achieving the full therapeutic potential of this unique hormone. With ongoing effort and scientific study, the road to improving diabetes management with adiponectin-based therapy holds enormous potential.
This entry is adapted from the peer-reviewed paper 10.3390/life13112213
References
- Ramakrishnan, N.; Auger, K.; Rahimi, N.; Jialal, I. Biochemistry, Adiponectin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23.
- Barbe, A.; Bongrani, A.; Mellouk, N.; Estienne, A.; Kurowska, P.; Grandhaye, J.; Elfassy, Y.; Levy, R.; Rak, A.; Froment, P.; et al. Mechanisms of Adiponectin Action in Fertility: An Overview from Gametogenesis to Gestation in Humans and Animal Models in Normal and Pathological Conditions. Int. J. Mol. Sci. 2019, 20, 1526.
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769.
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339.
- Liu, Z.; Xiao, T.; Peng, X.; Li, G.; Hu, F. APPLs: More than Just Adiponectin Receptor Binding Proteins. Cell. Signal. 2017, 32, 76–84.
- Goldstein, B.J.; Scalia, R. Adiponectin: A Novel Adipokine Linking Adipocytes and Vascular Function. J. Clin. Endocrinol. Metab. 2004, 89, 2563–2568.
- Delaigle, A.M.; Senou, M.; Guiot, Y.; Many, M.-C.; Brichard, S.M. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: In vivo and in vitro studies. Diabetologia 2006, 49, 1311–1323.
- Krause, M.P.; Liu, Y.; Vu, V.; Chan, L.; Xu, A.; Riddell, M.C.; Sweeney, G.; Hawke, T.J.; Sente, T.; Van Berendoncks, A.M.; et al. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am. J. Physiol. Physiol. 2008, 295, C203–C212.
- Guo, Z.; Xia, Z.; Yuen, V.G.; McNeill, J.H. Cardiac expression of adiponectin and its receptors in streptozotocin-induced diabetic rats. Metabolism 2007, 56, 1363–1371.
- Diep Nguyen, T.M. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136.
- Fantuzzi, G. Adiponectin and inflammation: Consensus and controversy. J. Allergy Clin. Immunol. 2008, 121, 326–330.
- Guenther, M.; James, R.; Marks, J.; Zhao, S.; Szabo, A.; Kidambi, S. Adiposity distribution influences circulating adiponectin levels. Transl. Res. 2014, 164, 270–277.
- Bahreini, M.; Ramezani, A.-H.; Shishehbor, F.; Mansoori, A. The Effect of Omega-3 on Circulating Adiponectin in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. J. Diabetes 2018, 42, 553–559.
- Barbosa, M.M.d.A.L.; de Melo, A.L.T.R.; Damasceno, N.R.T. The benefits of ω-3 supplementation depend on adiponectin basal level and adiponectin increase after the supplementation: A randomized clinical trial. Nutrition 2017, 34, 7–13.
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M.; et al. Concurrent Aerobic and Resistance Training Has Anti-Inflammatory Effects and Increases Both Plasma and Leukocyte Levels of IGF-1 in Late Middle-Aged Type 2 Diabetic Patients. Oxid. Med. Cell. Longev. 2017, 2017, 3937842.
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2017, 7, 57–62.
- Ziemke, F.; Mantzoros, C.S. Adiponectin in insulin resistance: Lessons from translational research. Am. J. Clin. Nutr. 2010, 91, 258S–261S.
- Lee, T.H.; Christie, B.R.; van Praag, H.; Lin, K.; Siu, P.M.-F.; Xu, A.; So, K.-F.; Yau, S.-Y. AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis. Int. J. Mol. Sci. 2021, 22, 2068.
- Krause, M.P.; Milne, K.J.; Hawke, T.J. Adiponectin—Consideration for its Role in Skeletal Muscle Health. Int. J. Mol. Sci. 2019, 20, 1528.
- Mancuso, P.; Mancuso, P.; Bouchard, B.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10, 137.
- Adamia, N.; Virsaladze, D.; Charkviani, N.; Skhirtladze, M.; Khutsishvili, M. Effect of metformin therapy on plasma adiponectin and leptin levels in obese and insulin resistant postmenopausal females with type 2 diabetes. Georgian Med. News 2007, 145, 52–55.
- Riera-Guardia, N.; Rothenbacher, D. The effect of thiazolidinediones on adiponectin serum level: A meta-analysis. Diabetes Obes. Metab. 2008, 10, 367–375.
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792.
- Duan, X.; Zhou, M.; Zhou, G.; Zhu, Q.; Li, W. Effect of metformin on adiponectin in PCOS: A meta-analysis and a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 61–67.
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749.
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946.
- Hopkins, T.A.; Ouchi, N.; Shibata, R.; Walsh, K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 2007, 74, 11–18.
- Raman, K.; Rajagopal, K.; Swaminathan, G.; Jupudi, S.; Dhama, K.; Barua, R.; Bin Emran, T.; Osman, H.; Khandaker, M.U. A Critical Review on the Potency of Phytoconstituents in the Management of COVID-19. J. Pure Appl. Microbiol. 2023, 17, 1320–1340.
- Laustsen, P.G.; Michael, M.D.; Crute, B.E.; Cohen, S.E.; Ueki, K.; Kulkarni, R.N.; Keller, S.R.; Lienhard, G.E.; Kahn, C.R. Lipoatrophic diabetes in Irs1−/−/Irs3−/− double knockout mice. Genes Dev. 2002, 16, 3213–3222.
- Søvik, O.; Vestergaard, H.; Trygstad, O.; Pedersen, O. Studies of insulin resistance in congenital generalized lipodystrophy. Acta Paediatr. Suppl. 1996, 413, 29–37.
- Moitra, J.; Mason, M.M.; Olive, M.; Krylov, D.; Gavrilova, O.; Marcus-Samuels, B.; Feigenbaum, L.; Lee, E.; Aoyama, T.; Eckhaus, M.; et al. Life without white fat: A transgenic mouse. Minerva Anestesiol. 1998, 12, 3168–3181.
- Akter, S.; Tasnim, S.; Barua, R.; Choubey, M.; Arbee, S.; Mohib, M.M.; Minhaz, N.; Choudhury, A.; Sarker, P.; Mohiuddin, M.S. The Effect of COVID-19 on Gut Microbiota: Exploring the Complex Interplay and Implications for Human Health. Gastrointest. Disord. 2023, 5, 340–355.
- Cavusoglu, E.; Ruwende, C.; Chopra, V.; Yanamadala, S.; Eng, C.; Clark, L.T.; Pinsky, D.J.; Marmur, J.D. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur. Heart J. 2006, 27, 2300–2309.
- Sharma, K. The link between obesity and albuminuria: Adiponectin and podocyte dysfunction. Kidney Int. 2009, 76, 145–148.
- Liu, Z.; Gan, L.; Wu, T.; Feng, F.; Luo, D.; Gu, H.; Liu, S.; Sun, C. Adiponectin reduces ER stress-induced apoptosis through PPARα transcriptional regulation of ATF2 in mouse adipose. Cell Death Dis. 2016, 7, e2487.
- Rakatzi, I.; Mueller, H.; Ritzeler, O.; Tennagels, N.; Eckel, J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 2004, 47, 249–258.
- Lin, P.; Chen, L.; Li, D.; Liu, J.; Yang, N.; Sun, Y.; Xu, Y.; Fu, Y.; Hou, X. Adiponectin Reduces Glucotoxicity-Induced Apoptosis of INS-1 Rat Insulin-Secreting Cells on a Microfluidic Chip. Tohoku J. Exp. Med. 2009, 217, 59–65.
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363.
- Momen, F.; Barua, R.; Kabir, G. Comparative Analysis of in Vitro Antioxidant and Cytotoxic Activity of Unripe and Ripe Fruits of Solanum sisymbriifolium. Asian J. Agric. Food Sci. 2020, 8, 6.
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr. Rev. 2012, 33, 547–594.
- Cohen, K.E.; Katunaric, B.; Schulz, M.E.; SenthilKumar, G.; Young, M.S.; Mace, J.E.; Freed, J.K. Role of Adiponectin Receptor 1 in Promoting Nitric Oxide-Mediated Flow-Induced Dilation in the Human Microvasculature. Front. Pharmacol. 2022, 13, 875900.
- Okamoto, M.; Ohara-Imaizumi, M.; Kubota, N.; Hashimoto, S.; Eto, K.; Kanno, T.; Kubota, T.; Wakui, M.; Nagai, R.; Noda, M.; et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia 2008, 51, 827–835.
- Wijesekara, N.; Krishnamurthy, M.; Bhattacharjee, A.; Suhail, A.; Sweeney, G.; Wheeler, M.B. Adiponectin-induced ERK and Akt Phosphorylation Protects against Pancreatic Beta Cell Apoptosis and Increases Insulin Gene Expression and Secretion. J. Biol. Chem. 2010, 285, 33623–33631.
- Brown, J.E.; Conner, A.C.; Digby, J.E.; Ward, K.L.; Ramanjaneya, M.; Randeva, H.S.; Dunmore, S.J. Regulation of beta-cell viability and gene expression by distinct agonist fragments of adiponectin. Peptides 2010, 31, 944–949.
- Kubota, N.; Terauchi, Y.; Yamauchi, T.; Kubota, T.; Moroi, M.; Matsui, J.; Eto, K.; Yamashita, T.; Kamon, J.; Satoh, H.; et al. Disruption of Adiponectin Causes Insulin Resistance and Neointimal Formation. J. Biol. Chem. 2002, 277, 25863–25866.
- Yamauchi, T.; Kamon, J.; Waki, H.; Imai, Y.; Shimozawa, N.; Hioki, K.; Uchida, S.; Ito, Y.; Takakuwa, K.; Matsui, J.; et al. Globular Adiponectin Protected ob/ob Mice from Diabetes and ApoE-deficient Mice from Atherosclerosis. J. Biol. Chem. 2003, 278, 2461–2468.
- Talukder, E.U.; Momen, F.; Barua, R.; Sultana, S.; Yesmin, F.; Islam, M.S.; Bhuiyan, R.H. In vitro Assessment of Cytotoxic Activity of Hybrid Variety of Momordica charantia (Bitter Gourd). J. Phytopharm. 2020, 9, 445–448.
- Bhuiyan, R.H.; Barua, R.; Talukder, E.U.; Islam, M.S.; Yesmin, F.; Chakma, K.; Kabir, G. Nutritional Analysis and Phytochemical Evaluation of Bitter Gourd (Momordica charantia) from Bangladesh. Asian J. Agric. Food Sci. 2020, 8, 2.
- Najnin, R.A.; Shafrin, F.; Polash, A.H.; Zaman, A.; Hossain, A.; Taha, T.; Ahmed, R.; Tuli, J.F.; Barua, R.; Sajib, A.A.; et al. A diverse community of jute (Corchorus spp.) endophytes reveals mutualistic host–microbe interactions. Ann. Microbiol. 2014, 65, 1615–1626.
- Barua, R.; Sultana, S.; Talukder, E.U.; Chakma, K.; Hasan, C.M.M.; Islam, M.S. Antioxidant and Cytotoxic Activity of Crude Flavonoid Fraction from the Fruits of Hybrid Variety of Momordica charantia (Bitter Gourd). Br. J. Pharm. Res. 2014, 4, 778–786.
- Chowdhury, W.K.; Arbee, S.; Debnath, S.; Bin Zahur, S.M.; Akter, S.; Karim, A.K.M.R.; Mohib, M.M.; Tisha, A.; Sagor, A.T.; Mohiuddin, S. Potent Role of Antioxidant Molecules in Prevention and Management of Skin Cancer. J. Clin. Exp. Dermatol. Res. 2017, 8, 1000393.
- Motegi, M.; Himeno, T.; Nakai-Shimoda, H.; Inoue, R.; Ozeki, N.; Hayashi, Y.; Sasajima, S.; Mohiuddin, M.S.; Asano-Hayami, E.; Kato, M.; et al. Deficiency of glucagon gene-derived peptides induces peripheral polyneuropathy in mice. Biochem. Biophys. Res. Commun. 2020, 532, 47–53.
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.-W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198.
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid-Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025.
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30.
- Biolo, A.; Shibata, R.; Ouchi, N.; Kihara, S.; Sonoda, M.; Walsh, K.; Sam, F. Determinants of Adiponectin Levels in Patients With Chronic Systolic Heart Failure. Am. J. Cardiol. 2010, 105, 1147–1152.
This entry is offline, you can click here to edit this entry!
