Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Sodium–glucose cotransporter-2 (SGLT-2) inhibitors, also called gliflozins or flozins, are a class of drugs that have been increasingly used in the management of type 2 diabetes mellitus (T2DM) due to their glucose-lowering, cardiovascular (CV), and renal positive effects.
- SGLT-2 inhibitors (gliflozins)
- T2DM
- cardiovascular
- ketogenesis
1. Introduction
SGLT-2 (sodium–glucose cotransporter-2) inhibitors, often referred to as gliflozins or flozins, achieved a significant milestone in 2012 with approval by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) for the treatment of type 2 diabetes mellitus (T2DM) [1,2,3]. This moment marked a paradigm shift in addressing elevated blood glucose levels. Within this context, the triad of endorsed SGLT-2 inhibitors, dapagliflozin (DAPA), canagliflozin (CANA), and empagliflozin (EMPA), emerged as pivotal components in the management of hyperglycemia. By concurrently modulating renal glucose reabsorption and enhancing urinary glucose excretion, this trio presented a potent therapeutic avenue for ameliorating the impact of T2DM [4,5,6,7].
While initially pursued for cardiovascular safety validation, SGLT2 inhibitors defied expectations in cardiovascular outcome trials (CVOTs) like EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in T2DM Patients), CANVAS (Canagliflozin Cardiovascular Assessment Study), DECLARE-TIMI-58 (Dapagliflozin Effect on Cardiovascular Events−Thrombolysis In MI (Myocardial Infarction) 58), VERTIS CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular outcomes), and SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with T2DM and Moderate Renal Impairment Who Are at Cardiovascular Risk). These trials surprisingly unveiled SGLT2 inhibitors’ ability to significantly reduce major adverse cardiovascular events (MACEs) compared to placebos. Notably, the 2015 EMPA-REG OUTCOME trial first demonstrated this protective effect, showcasing a 14% MACE reduction, 34% lower all-cause mortality, and 35% fewer heart failure (HF) hospitalizations [8]. Subsequent studies, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in HF) and EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic HFrEF (Heart Failure with Reduced Ejection Fraction)), revealed SGLT2 inhibitors’ transformative potential in HF treatment for patients with a reduced EF (ejection fraction) <40%, regardless of T2DM, decreasing hospitalizations, and mortality [9,10]. The EMPEROR-Preserved trial (Empagliflozin Outcome Trial in Patients with Chronic HFpEF (Heart Failure with Preserved Ejection Fraction)) in 2021 extended these benefits to chronic HF patients, irrespective of EFs, emphasizing SGLT2 inhibitors’ expanding role in enhancing prognosis [11].
In addition to that, the discovery of gliflozins’ nephroprotective effect has greatly impacted clinical practice as well. Diabetes is associated with microvascular damage, often culminating in chronic kidney disease (CKD) for around 40% of patients. Investigated in CVOTs, gliflozins effectively mitigate declines in the glomerular filtration rate (GFR), delay microalbuminuria, and hinder proteinuria progression, favoring both diabetic and nondiabetic patients. Recent EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin) trial data show SGLT2 inhibitors’ efficacy in nephropathy, even with a diminished eGFR (estimated glomerular filtration rate). Consequently, SGLT2 inhibitors assume a pivotal role in reducing the progression to end-stage renal disease (ESRD) among patients with CKD [12].
Beyond the aforementioned effects, SGLT-2 inhibitors have a pro-ketogenic effect that has been associated with their potential to increase the production of ketone bodies, such as BHB (β-hydroxybutyrate) [13]. This pro-ketogenic effect holds promising implications for cardiovascular events such as stroke and HF, as well as for retarding the progression of chronic diseases, such as atherosclerosis and CKD [14,15].
2. Mechanism of Action of SGLT-2 Inhibitors
Sodium–glucose cotransporters (SGLTs) are a class of transmembrane proteins that share a common transport mechanism in which extracellular sodium binding causes a gate to open and traps glucose from outside of the cell. The transporter then shifts, releasing sodium and glucose into the cytoplasm. The protein reverts to its initial conformation at the completion of the process. The two most common SGLTs are SGLT-1 (sodium–glucose cotransporter-1) and SGLT-2 (sodium–glucose cotransporter-2) [26,27].
The SGLT2 transporter is predominantly expressed in the epithelial cells of the renal proximal convoluted tubule. Although SGLT2 has a low affinity for glucose, it demonstrates a remarkable capacity for renal glucose reabsorption [28]. The process is primarily sodium-dependent, with SGLT2 and SGLT1 exhibiting ratios of 1:1 and 2:1, respectively. While SGLT1 is responsible for only approximately 10% of the tubular glucose reabsorption, SGLT2 handles the majority of the reabsorption. This capacity for reabsorbing filtered glucose in the kidneys is an extremely efficient energy conservation mechanism. Apart from the kidneys, SGLT2 expression has been detected in other organs such as the brain, liver, thyroid, muscles, and heart, while SGLT1 expression has been reported in the intestine, trachea, kidney, heart, brain, testes, and prostate. The high capacity of renal glucose reabsorption, especially by SGLT2 has led to the development of SGLT2 inhibitors as a treatment for T2DM [26].
Within this framework, SGLT-2 inhibitors function by obstructing the activity of the SGLT-2 protein, which is prominently present in the proximal convoluted tubules of the kidneys. This action effectively prevents the reabsorption of filtered glucose from the tubular lumen [29]. Their mechanism of action is based on the renal excretion of glucose, causing glucosuria, and is independent of insulin action, thus reducing hypoglycemia, weight gain, and liver disease [30].
An improved comprehension of SGLT2 inhibitors and their impact on insulin sensitivity holds promise for enhancing treatments for T2DM and metabolic disorders. This insight may also illuminate the intricate interplay between insulin sensitivity and glucose balance, which is vital for maintaining metabolic health. A schematic illustration that indicates the mechanism of action of SGLT-2 inhibitors in detail is shown in Figure 1.
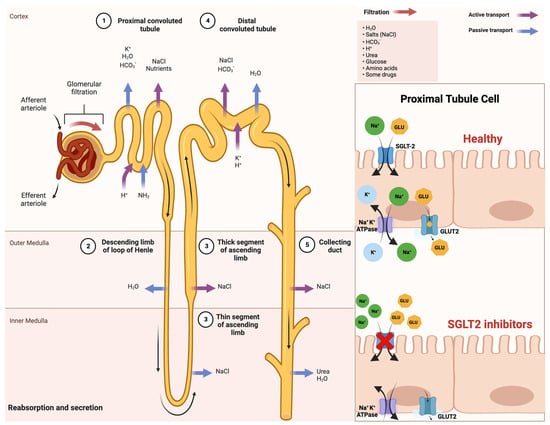
Figure 1. Comprehensive schematical depiction of the mechanism of action of SGLT2 inhibitors. Abbreviations: SGLT2—sodium−glucose cotransporter 2; Na+—sodium cations; K+—potassium cations; H2O—oxygen hydride (water); HCO3−—bicarbonate ions; NaCl—sodium chloride (salt); H+—hydrogen ions; NH3—ammonia; GLU—glucose; ATPase—adenosine triphosphatase (enzyme); GLUT2—glucose transporter 2. Created with BioRender.com and accessed on 17 April 2021.
3. Synergism of SGLT-2 Inhibitors and Ketogenic Diet: Benefits and Risks
3.1. Positive Synergistic Effect of SGLT-2 Inhibitors and Ketogenic Diet: Benefits
Ketogenic diets and SGLT2 inhibitors are two highly promising therapeutic approaches for treating T2DM and other complications. In recent years, there has been a noticeable increase in interest in both of these strategies due to their potential synergistic health merits, such as weight reduction, improved insulin sensitivity, and decreased cardiovascular risk [59,60,61].
As previously noted, SGLT-2 inhibitors are a group of oral anti-diabetic medications that prevent the kidneys from reabsorbing glucose, enhance urine glucose excretion, and lower blood sugar levels [62]. On the other hand, the ketogenic diet, characterized by its low-carbohydrate and high-fat composition [63,64], consisting approximately of 70–80% fat, 10–20% protein, and 5–10% carbohydrates, induces ketosis, a metabolic state in which the body primarily uses fat for energy production by producing ketones rather than glucose [65]. Both approaches independently promote weight loss and improve glycemic control, which are crucial for managing these conditions [66].
In more detail, SGLT-2 inhibitors exert their weight loss effects through the increased urinary excretion of glucose, leading to a calorie deficit and a subsequent reduction in body weight. This weight loss is predominantly due to a decrease in adipose tissue, which is beneficial since excessive adipose tissue is primarily associated with insulin resistance and inflammation in T2DM [68].
To amplify these therapeutic benefits, the combined use of SGLT-2 inhibitors with a ketogenic diet may prove to be an innovative tactic. In fact, recent studies have suggested that combining SGLT-2 inhibitors with ketogenic diets may have additive effects on glucose control and other metabolic parameters [75]. Taking into consideration the fact that both interventions can lead to a decrease in HbA1c [76,77], body weight [68,78], and blood pressure [79,80,81], it seems reasonable to say that the combination of the two would result in even greater reductions in HbA1c, body weight, and blood pressure than either treatment alone, thereby offering a more effective approach to managing T2DM and obesity. On top of that, considering the anti-inflammatory effects of both treatments [82,83], their simultaneous use may lower the risk of chronic inflammation and associated CVD, bringing about superior outcomes.
Numerous assertions have been put forth regarding the neuroprotective advantages attributed to both ketogenic diets and SGLT-2 inhibitors, further emphasizing their potential impact on neurological health [83,84,85]. While the only well-established use of a ketogenic diet is for reducing seizures in pediatric epilepsy [86], its ability to produce ketone bodies that serve as alternative fuels for brain metabolism is key to maintaining mitochondrial function, ATP (the source of energy for use and storage at cellular level) production, and neuronal survival.
3.2. Negative Synergistic Effect of SGLT-2 Inhibitors and Ketogenic Diet: Risks
Very-low-carbohydrate diets, also known as ketogenic diets, were initially popular fad diets for weight reduction [91,92], but they are now used in medicine to treat diabetes, epilepsy, and obesity [91,92,93,94,95]. A rise in the concurrent use of ketogenic diets and SGLT-2 inhibitors to control weight in diabetic patients is due to the increased use of SGLT-2 inhibitors in the management of diabetes [28]. In spite of the potential synergistic benefits that might occur, the combination of these inhibitors and ketogenic diets may also pose certain risks [75].
Given that, one major concern is the increased risk of euglycemic diabetic ketoacidosis (euDKA, DKA), a potentially life-threatening emergency and potentially fatal condition characterized by the excessive production of ketone bodies, including BHB, and a subsequent decrease in blood pH [66,75,96]. DKA can be specifically distinguished by the triad of euglycemia (blood glucose less than 250 mg/dL) in the presence of severe metabolic acidosis (arterial pH less than 7.3, serum bicarbonate less than 18 mEq/L) and ketonemia [97]. It should be highlighted that euDKA can appear in patients with both T1DM and T2DM. Because euglycemic ketosis can be brought on by either a ketogenic diet or SGLT-2 inhibitors alone [21,98,99,100], there is probably a greater possibility that it will occur when both therapies are combined.
Another potential risk is the possibility that the combination of SGLT-2 inhibitors and a ketogenic diet could result in dehydration [96]. SGLT-2 inhibitors, through their mechanism of action, may cause increased glucose excretion in the urine, along with an increased excretion of water and electrolytes, including sodium [81]. The ketogenic diet can also lead to increased water and electrolyte loss due to the diuretic effect of ketones [93]. As a result, combining these two therapies may increase the risk of both dehydration and electrolyte imbalances.
It has also been suggested that the combination of SGLT-2 inhibitors and a ketogenic diet may result in gastrointestinal (GI) disturbances [96]. Nevertheless, both treatments can cause GI side effects independently, such as nausea, vomiting, and diarrhea [30,107]. Consequently, when using SGLT-2 inhibitors and following a ketogenic diet, patients should be evaluated for any signs of GI symptoms.
Other adverse effects may include infections, such as urinary and genital infections. To date, there is no concrete proof that using SGLT-2 inhibitors and a ketogenic diet can cause infections. What is known is that SGLT-2 inhibitors have been associated with an increased risk of genital and urinary tract infections owing to their mechanism of action [108], which involves reducing glucose reabsorption in the kidneys and provoking glycosuria. This can create an environment in the urinary tract that is more conducive to bacterial growth.
The data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years, were analyzed in this context as well. Upon enrollment in the Biobank, 70,684 participants completed a one-time self-reported 24 h diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that their diet during the 24 h reporting period met the study’s definition of an LCHFD. These participants were matched by age and sex with 1220 individuals who reported eating a standard diet. The result was that the average age of the study population was 54 years, and 73 percent were women. The mean BMI was 27.7 for those on the LCHFD and 26.7 for those on the standard diet.
4. Clinical Applications of SGLT-2 Inhibitors as Ketogenic Agents
4.1. Type 2 Diabetes Mellitus
Over the past two decades, the treatment of T2DM has undergone continuous refinement, representing an ever-evolving discipline within the medical sciences. The frequency of severe catastrophic effects, including amputations, renal failure requiring dialysis, and blindness from retinopathy, has considerably declined as a result of therapeutic advancements. Drug development has achieved therapeutic objectives. That being said, millions of people with T2DM may benefit from the glycemic and nonglycemic effects of the inhibitors of SGLT-2, a class of drugs with remarkable versatility.
The selection of pharmacotherapy for T2DM is largely dependent on the comorbidity of CVD and CKD. This is in accordance with the joint recommendations of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), initially published in 2018 and subsequently revised in late 2022 [122,123]. The guidelines established by the European Society of Cardiology (ESC) in 2023 also have a vital impact on influencing these decisions [124].
The current guidelines, developed by the SCORE2-Diabetes Working Group and the ESC Cardiovascular Risk Collaboration, provide evidence-based suggestions for managing cardiovascular risk in individuals with diabetes and offer guidance on how to tackle atherosclerotic cardiovascular disease (ASCVD) in patients with diabetes. The guidelines introduce a novel, 10-year cardiovascular risk assessment tool, SCORE2-Diabetes, for patients with T2DM who do not have ASCVD or significant target organ damage (TOD). This expanded version of the established SCORE2 prediction model for T2DM estimates the 10-year probability of fatal and nonfatal cardiovascular events (like an MI/heart attack or stroke) based on the patient’s individual characteristics. The SCORE2-Diabetes score is a useful tool for clinical decision making for patients with T2DM at different risk levels—low, moderate, high, or very high—but without clinically evident ASCVD or severe TOD [124,125].
4.2. Obesity
In the last 15 years, the prevalence of obesity has doubled, and it is becoming a growing health problem that has gained pandemic dimensions. Based on data from the World Health Organization, in 2016, roughly 40% of the global adult population, equating to 1.9 billion individuals, were affected by overweight or obesity, with 650 million among them exhibiting a more severe form of obesity [126].
In addition to the cornerstone of overweight and obesity, which is lifestyle modification, adjuvant pharmacotherapies, such as SGLT2 inhibitors, instantly reduce body weight by causing the kidneys to excrete glucose, which results in calorie loss. Amounts of 60–100 g of glucose per day may be eliminated in the urine as a result of SGLT2 antagonism, which functions in a glucose-dependent way [68].
In this context, numerous studies have consistently demonstrated the weight loss efficacy associated with SGLT2 inhibitor therapy in patients with T2DM, regardless of whether these individuals are administered SGLT2 inhibitors alone or in conjunction with other glucose-lowering therapies [68].
In compliance with findings from certain meta-analyses, all SGLT2 inhibitor treatments have demonstrated reductions in body weight of approximately 1.5–2 kg compared to a placebo [127,128,129,130], with the magnitude of this effect being dose-dependent [131]. Clinical evidence spanning up to four years indicates that the capacity of these drugs to elicit reductions in body weight is sustained over the course of treatment [132,133,134].
4.3. Heart Failure
Heart failure (HF) affects approximately 40 million individuals globally. The prevalence of HF is escalating to epidemic proportions, which can be attributed in part to the worldwide burden of cardiovascular risk factors and the aging of populations [140,141,142,143,144]. Despite substantial progress made in medical and device-based therapies for HF patients [145] in recent decades, mortality rates remain alarmingly high [146].
Pharmacotherapy continues to be the foundation of HF treatment, and various potent novel therapeutic avenues have recently emerged [147]. These include angiotensin-neprilysin receptor inhibitors (ARNi) [148], SGLT-2 inhibitors, omecamtiv mecarbil (INN) [149], and vericiguat [150]. Among these options, SGLT-2 inhibitors, as ketogenic agents, have arguably demonstrated the most remarkable and consistent benefits across the HF spectrum, coupled with an outstanding safety profile [151].
It has been hypothesized that the oxidation of ketone bodies serves as an additional fuel source, which is highly energy-efficient [152] and efficiently extracted by the heart [9,10,153]. Furthermore, patients without T2DM with HFrEF who received a continuous infusion of BHB showed increases in CO (cardiac output), LVEF (left ventricular ejection fraction), and myocardial oxygen consumption without altering the myocardial external energy efficiency [154].
Until now, multiple studies have shown that SGLT2 inhibitors reduce HF-related mortality [154,162,163]. Increased ketogenesis as a result of SGLT-2 inhibitors may significantly change energy metabolism in cardiomyocytes (or cardiac cells), reducing energy deficits in the myocardium [164,165]. In HF, cardiomyocytes have a lower ability to use glucose or fatty acids. Therefore, ketones have become a more important fuel source, providing energy in a highly efficient manner with significant uptake by myocytes [165]. A heart deprived of oxygen can benefit from ketone body oxidation, as, when combined with fatty acids, they are more efficient in terms of oxygen utilization [165,166]. This mechanism has the potential to serve as an efficacious therapeutic strategy for addressing the metabolic aspect of HF by ameliorating O2 consumption and curtailing reactive oxygen species (ROS) production through the use of SGLT2 inhibitors. As a result, it could lead to more effective cardiac functioning and impede the progression of HF [154,162,165].
Further, the outcomes of EMPAREG OUTCOME, CANVAS, and DECLARE-TIMI 58 trials were summarized in a meta-analysis. It was shown that while there was no difference in primary prevention [173], T2DM patients with atherosclerotic CVD experienced better cardiovascular outcomes. These results support the 2020 ESC guidelines’ suggestion that individuals with T2DM and established CVD obtain SGLT2 inhibitors as their first treatment [174]. Several additional or exploratory end points, such as those related to HF and renal disease, were also gathered from these trials.
4.4. Kidney Disease
Similar to the heart, the kidney is also a highly energetically and metabolically active organ. Hence, in cases of kidney failure and CKD, ketone bodies offer a promising oxygen-efficient energy source that can prevent kidney hypoxia [162,185]. Utilizing ketones as fuel reduces the risk of cell apoptosis, fibrosis, and the development of diabetic nephropathy, commonly known as diabetic kidney disease (DKD) [154]. SGLT2 inhibition induces ketogenesis, which then is believed to affect mTORC1 (the mammalian target of rapamycin complex 1) signaling. The inhibition of mTORC1 by ketones may subsequently prevent kidney damage, thus providing renoprotection [154,186].
Moreover, SGLT2 inhibitors activate the SIRT1 (sirtuin-1) gene regulator, which is responsible for gluconeogenesis and therefore ketogenesis. Certain genetic variations of SIRT1 have been linked to an increased susceptibility to developing diabetic nephropathy. It has specifically been suggested that the reno-protective effects of SGLT2 inhibitors may be due to their ability to stimulate SIRT1 genes [154,186,187].
Regarding kidney disease, three cardiovascular outcome trials in patients with T2DM, with or without prevalent CVD, showed that SGLT2 inhibitors might lower a composite of renal outcomes by 40–70%, including the requirement for dialysis and/or transplantation, the development of macroalbuminuria, the doubling of blood creatinine, and kidney mortality [8,163,182]. In regard to CKD end points, a CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) in patients with DKD and DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in CKD) in patients with any form of CKD, as well as other minor studies such as DELIGHT (Delay Of Impaired Glucose Tolerance By A Healthy Lifestyle Trial Abbreviation), DERIVE (A Study to Evaluate the Effect of Dapagliflozin on Blood Glucose Level and Renal Safety in Patients with T2DM), and DIAMOND (Effects of Dapagliflozin on Proteinuria in Non-Diabetic Patients With CKD), were designed in populations with or without diabetes at risk for or suffering from cardiac or renal pathologies [9,10,181,190,191,192,193,194,195,196].
4.5. Metabolic-Associated Fatty Liver Disease (MAFLD)
Metabolic-associated fatty liver disease (MAFLD) is the most prevalent liver disease globally, and more than 50% of people diagnosed with T2DM also have MAFLD. These two conditions are linked in a bidirectional pathological relationship, where MAFLD increases the likelihood of developing T2DM, while T2DM contributes to and accelerates the progression of MAFLD [198]. More than 30% of MAFLD patients develop NASH, which raises the risk of developing cirrhosis and HCC (hepatocellular cancer) [199,200,201]. The underlying pathophysiology of MAFLD has not yet been fully established despite its high frequency and possible clinical consequences, and there is no agreement on the typical diagnosis and course of action for either MALFD or NASH. Patients with both NASH and T2DM have compromised hepatic function as a result of chronic inflammation and the ensuing structural alterations brought on by hepatic fat buildup, which limits their options for anti-diabetic therapy [202].
The SGLT2 inhibitor, canagliflozin (CANA), induces significant transcriptional reprogramming and metabolic shifts, characterized by increased fatty acid oxidation, reduced hepatic steatosis, and elevated levels of the hepatokine FGF21. Interestingly, the induction of lipid oxidation and ketogenesis by CANA seems to proceed independently of FGF21, yet this hepatokine plays a critical role in the activation of lipolysis and adiposity reduction, illustrating a complex, dual pathway of metabolic regulation that mirrors a fasting-like metabolic paradigm [204].
When considered in the context of MAFLD, SGLT2 inhibitors’ ketogenic effect could provide therapeutic benefits. Insufficient hepatic ketogenesis, a major pathogenic mechanism implicated in MAFLD, influences the expression of genes associated with de novo lipogenesis, consequently leading to excess lipid accumulation in hepatocytes. Decreased ketone body levels can escalate mitochondrial stress and exacerbate triglyceride accumulation, expediting the progression of MAFLD to NASH through heightened oxidative stress [200,203,205,206].
In the context of the previously discussed mechanisms, SGLT2 inhibitors stimulate SIRT1 expression, thereby promoting gluconeogenesis and, in effect, amplifying ketogenesis. This process holds potential advantages not only for managing kidney dysfunction but also for MAFLD treatment. An elevated SIRT1 expression initiates an increase in fatty acid oxidation and a decrease in hepatic lipid storage by facilitating TAG catabolism. As such, SIRT1 activation emerges as a potential pathway for designing therapeutic strategies, potentially increasing the effectiveness of SGLT2 inhibitors in managing metabolic conditions. [154,187,209].
5. Conclusions
In conclusion, SGLT-2 inhibitors are a promising class of medications for the treatment of T2DM and other metabolic diseases. Their unique mechanism of action, which is independent of insulin, makes them a valuable addition to the armamentarium of healthcare professionals. However, their use must be monitored, and patients have to be educated on both the benefits and potential risks of SGLT-2 inhibitors. Ongoing research will continue to shed light on the complex interplay between glucose metabolism, insulin sensitivity, and the gut microbiome, which may ultimately lead to more effective and personalized treatments for metabolic diseases.
Due to their complex and multidirectional mechanism of action, this originally antidiabetic group of drugs has been successfully used to treat patients with HF and patients with CKD as well [236,237]. Moreover, their therapeutic potential seems to be even wider than the indications studied so far.
Ongoing clinical trials evaluating the effects of SGLT-2 inhibitors, particularly canagliflozin, on cardiorenal outcomes in patients with DKD have shown promising results. These findings suggest that canagliflozin may emerge as an effective and safe therapy for improving outcomes in both diabetic and nondiabetic kidney disease [238]. As more data become available from these and other ongoing trials, the role of SGLT-2 inhibitors in the management of DKD is expected to become clearer and may lead to the incorporation of these drugs as a cornerstone of therapy for patients with this debilitating condition [96,97,101,238,239,240].
The synergism of SGLT-2 inhibitors and ketogenic diets represents a promising therapeutic approach for managing T2DM and obesity. By promoting weight loss and improving glycemic control, this combination may offer enhanced clinical benefits compared to either intervention alone. However, potential risks, such as the increased risk of DKA and AKI (acute kidney injury), must be carefully considered and monitored [66,96,97,98,101,240]. Further research is required to elucidate the optimal combination of these treatments and to develop strategies for mitigating their associated risks. Ultimately, an individualized approach to therapy, considering each patient’s unique clinical circumstances, will be essential in maximizing the therapeutic potential of SGLT-2 inhibitors and ketogenic diets in the management of T2DM and obesity.
Finally, with the incredibly high incidence of T2DM in the current population of emergency department patients, it is critical for clinicians to understand the possible complications of the treatment of this disease. SGLT-2 inhibitor medications are becoming very common encounters on patient medication lists, and clinicians should be aware of how these medications, alone or combined with dietary modifications, can result in significant pathology and even mortality if not appropriately treated [241].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10110465
This entry is offline, you can click here to edit this entry!
