Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Marine cyanobacteria are an ancient group of photosynthetic microbes dating back to 3.5 million years ago. They are prolific producers of bioactive secondary metabolites.
- marine cyanobacteria
- climate change
- nitrogen fixation
1. Introduction
Cyanobacteria may be described as the pioneers of the planet Earth and are a one-of-a-kind gift of nature. The historical record dates the existence of cyanobacteria back to over 3.5 million years ago (MYA). They possess a number of natural qualities that make them suitable for several biotechnological applications [1][2][3]. Cyanobacteria (blue-green algae) are Gram-negative eubacteria. They are widely distributed throughout terrestrial, fresh water, wastewater, and marine habitats due to their immense morphological and physiological diversity [2][4]. Cyanobacteria come in a wide variety of morph types, such as species that are unicellular, surface-attached, as well as filamentous colony- and mat-forming. In the last few decades, the advent of ultrastructural and molecular methods has been evident as per the progress in taxonomic classification, the dominant tool for evaluating cyanobacterial diversity. These methods explain many yet unclear structures, adaptations, and phylogenetic relations within different clusters. The molecular methods rely on 16S rRNA gene sequencing as a standard, notably at the genus level. The 16S rRNA gene sequencing clusters closely correlate with traditional cyanobacterial taxa, which can be identified by various phenotypic characteristics [5][6]. Several species form significant symbiotic relationships with other micro- and macro-eukaryotes [7][8]. As life evolved on Earth, cyanobacteria played a pivotal role as the first organisms to engage in oxygenic photosynthesis [9]. In addition to their evolutionary niche, cyanobacteria have also played vital roles in the biogeochemical cycle of carbon (C) and nitrogen (N). An estimated 20–30% of the organic carbon present on Earth is derived from photosynthetic carbon fixation by cyanobacteria. Cyanobacteria also represent the primary N2-fixing microorganisms in marine environments [10][11][12].
Natural products have historically been used to inspire and develop new medications, and this remains one of the most successful methods for the discovery of small molecules for drug development [13]. Cyanobacteria are a unique source of small molecules, since they are thought to be one of the oldest forms of life on Earth and may thus constitute chemical factories with highly evolved secondary metabolite synthesis machinery. They have also been demonstrated to influence the biosynthesis of chemicals in marine invertebrates, such as sponges, ascidians, and shell-less mollusks, via endosymbiosis or diet-derived enrichment [13][14][15][16]. To date, more than 2000 secondary metabolites have been identified from marine cyanobacteria, especially Moorea, Lyngbya, and Okeania spp. [17][18]. Marine cyanobacteria have attracted the attention of many scientists in the fields of medicinal chemistry and pharmacology [19], as they demonstrate powerful biological activities such as antibacterial [20], antifungal [21], anticancer [22], and cytotoxic activities [23], as well as immunosuppression [24], anti-inflammatory [25], and antioxidant properties [26]. For instance, apratoxin D is a potent bioactive compound that is isolated from the marine cyanobacterium Lyngbya sp. and has demonstrated significant cytotoxicity against human lung cancer cells [27]. Four bioactive compounds, Dudawalamides A-D, isolated from Moorea producens were proven to be effective antiparasitic agents. All four of these compounds demonstrated potent activity, with IC50 ≥ 10 mM [28].
2. History and Existence
The organized structure and differentiation in the morphology of cyanobacteria, and their dominance in paleontological history [29], are due to the vital role that the unique cyanobacterial metabolism played in ancient ecosystems. This is also influenced by the selectivity of preservation and fossilization for particular environments or cyanobacterial taxa. The cyanobacterial fossil record is one of the oldest, reaching back to approximately 3500 Ma [30]. Fossil identification occurs by comparing old samples (established specimens existing in the herbarium) with modern forms in the natural population [30][31]. Scientists have devoted their efforts to documenting cyanobacteria across different eras (Figure 1). Several studies have documented that the most ancient in disputable fossil cyanobacteria were recorded in the Proterozoic Eon. Eoentophysalis belcherensis Hofmann was the first colonial coccoid microfossil present in this period. Their existence has been reported in intertidal and shallow subtidal environments on a carbonate platform in Canada [31][32]. Other fossils were recorded by Lanier (1989) as dating back to the Paleoproterozoic era. These fossils include vertically and horizontally oriented filaments, viz. Gunflintiaminuta, suggesting photoautotrophic cyanobacteria [33]. During the early Mesoproterozoic era, the cyanobacterial population, including entophysalidacean, oscillatoriacean, and nostocalean cyanobacteria, were widely spread across all possible ecological niches, from supratidal flats to open shelf marine environments [34][35]. The formation of distinct carbonate precipitates in this era helped fossilize a member of what was named the Archaeoellipsoides cyanobacterial genus in India [36]. Although they share many similarities with the extant Synechococcus genus, the samples show residues of akinetes resembling cyanobacteria Nostoc or Stigonema [29][37]. The late Mesoproterozoic era shows evidence of the existence of mat-dwelling and planktic chroococcacean cyanobacteria, with some cyanobacteria recognizable today dating back to the early Mesoproterozoic era. A distinctive feature of the evolution of cyanobacteria is the presence of a stalked cyanobacterium Polybessurus bipartitus [34]. The diversification of protists represents a critical event for developing the biosphere, and this has been documented as occurring in the era now named the Neoproterozoic era. Some new morphological varieties of cyanobacterial fossil debuted in this era, i.e., the remains of the stalked cyanobacterium Polybessurus Fairchild ex Green et al., which can sometimes be found in sediments [29]. In the near past, morphological criteria were solely used to classify cyanobacteria taxonomically; however, in the modern taxonomy, more adequate and complementary tools have been employed [38]. In the last few decades, the criteria of cyanobacterial taxonomic classification have radically changed after applying data obtained from electron microscopic studies along with phylogenetic analyses, mainly derived from molecular sequencing [39]. Modern techniques, such as the molecular method combined with traditional cyanobacterial morphological and ecological diversity studies, as well as the re-evaluation and redefinition of contemporary typical characters and markers of cyanobacterial entities in various biotopes, are inevitable taxonomic criteria for the primary classification. The molecular method used 16S rRNA gene sequencing as a standard, particularly at the genus level. The 16S rRNA gene sequencing clusters correspond to the traditional cyanobacterial taxa, which are identified by distinctive phenotypic features [5][6]. The combination of molecular methods with cyanobacterial taxonomy advanced important key aspects of cyanobacterial systematics as follows: Firstly, regarding various taxonomic units, the morphological characters should concur with their phylogenetic position. Secondly, phylogenetic relationships among cyanobacteria were discovered using combining molecular and phenotypic analyses, and a highly genetic variety was identified, particularly in coccoid and simple filamentous and pseudo-filamentous types [40]. Finally, a number of studies concluded that the fossil record of cyanobacteria, along with the modern methods of re-evaluation and redefinition of contemporary typical characters, helped scientists understand the complexity and diversity present in the history of evolution.
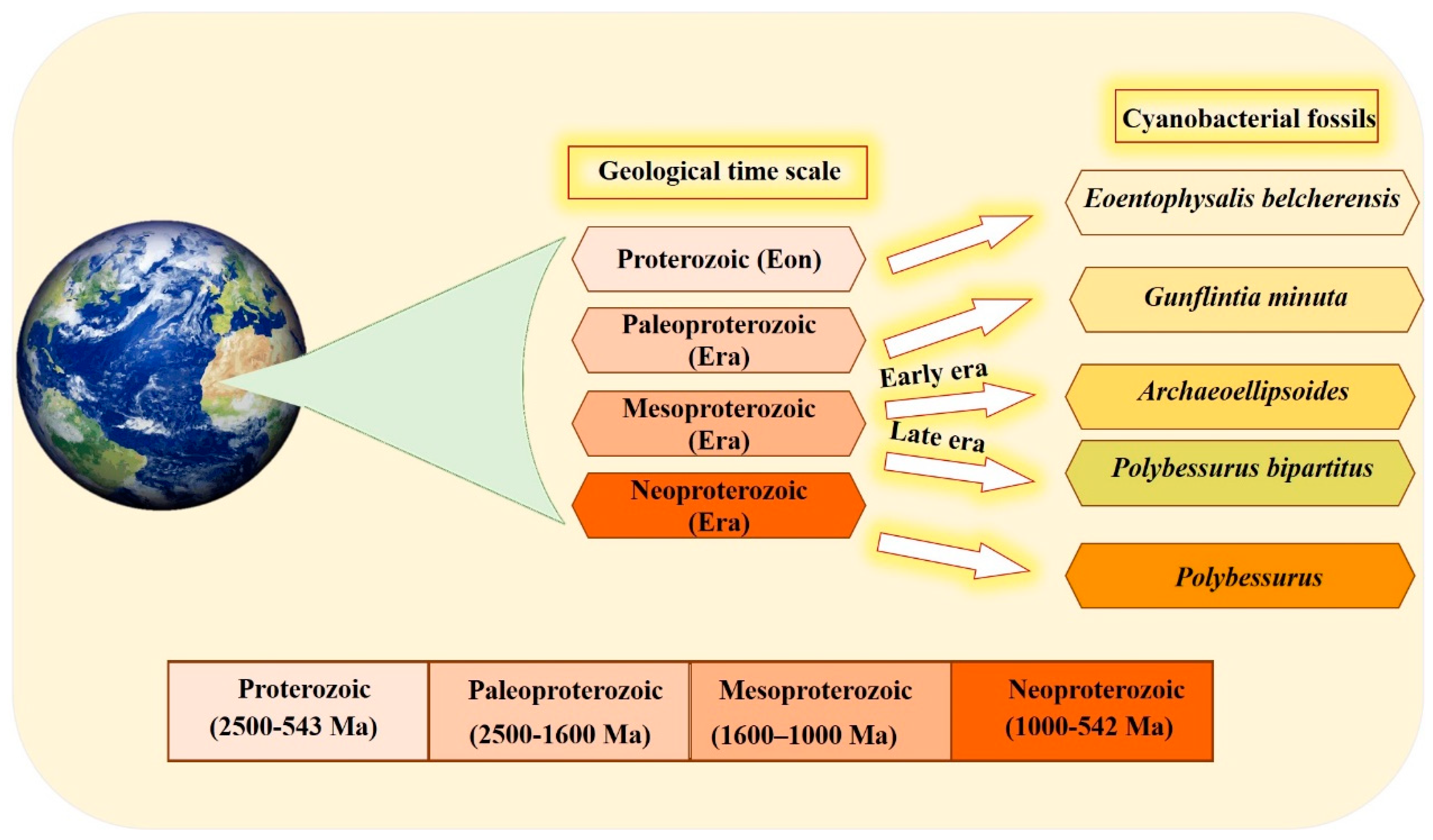
Figure 1. Timeline of the different cyanobacterial fossils recorded through different eras, mainly over the period ranging from 2500 to 542 million years ago, using a geological time scale.
3. Mechanism of Marine Cyanobacteria in Nitrogen Fixation
Cyanobacteria are recognized as the main N2-fixing microorganisms in the marine environment that participate in the universal nitrogen cycle [10][41]. N2-fixing cyanobacteria are grouped into filamentous non-heterocyst, unicellular cyanobacteria, and filamentous heterocyst-forming symbionts Figure 2 [10]. Regarding filamentous non-heterocyst, the genus Trichodesmium represents the most conspicuous marine organisms in terms of N2 fixing [42]. They are estimated to be responsible for approximately 50% of the global natural N2 fixation. Their nitrogen fixation process occurs during the day at temperatures ranging between 24–30°C, but stops at night due to inactivation and degradation of the nitrogenase enzyme [43][44]. Where N2 fixation occurs in differentiated cells of Trichodesmium spp., these cells consistently remain in clusters of about 3 to 20 [45]. The N products, such as ammonia (NH3), ammonium (NH4+), or the amino acid glutamine, are directly produced as a result of the reduction of N2 [46][47]. The following synthesis of glutamine and glutamate by a glutamine synthetase/glutamine oxoglutarate aminotransferase (GS/GOGAT) reaction also requires energy in the form of 1 ATP and 1 NADPH + H+. Thus, Trichodesmium’s capacity to fix nitrogen is highly reliant on the bioavailability of energy [48]. Unicellular N2-fixing cyanobacteria were first discovered through the amplification of nitrogenase genes and gene transcripts (mRNAs) from oceanic water samples [49]. According to recent studies, unicellular cyanobacteria (UCYN) have a high ability to fix N2 within a cell-size fraction below 10 μm. These unicellular cyanobacteria have been classified into three groups based on their nifH gene phylogeny: UCYN-A, UCYN-B, and UCYN-C [49][50][51]. In the UCYN-A group, cyanobacterial nitrogenase gene sequences were most tightly linked to sequences from the marine unicellular cyanobacterium Cyanothece sp. strain ATCC 51142 [52]. Despite numerous attempts, UCYN-A cyanobacteria have not been successfully cultivated [53]. UCYN-A cyanobacteria have genotypes that have not previously been identified in free-living cyanobacteria, and they lack the genetic ability for oxygenic photosynthesis. Furthermore, nitrogenase genes are most abundant in UCYN-A during the light period, and thus can fix N2 during daylight [51]. UCYN-B is a group of free-living unicellular cyanobacteria that fix N2 [54]. By studying the geographical populations of these free-living creatures, it may be concluded that they significantly contribute to the global N2 fixation process [55]. UCYN-B have only been identified in surface water and at 25% light depths (14 m) and is small in size (<10 μm) [56][57]. They fix N2 during the night [10]. In UCYN-B (Crocosphaera watsonii), nitrogenase enzyme synthesis begins just before nightfall to prepare for the upcoming N2 fixing activity during the night [58]. UCYN-C is a group of unicellular free-living cyanobacteria that fix N2 during the night [55][59]. It includes several cultivated cyanobacteria, such as Cyanothece sp. strain ATCC51142 and TW3 [60][61]. By 1993, Reddy and colleagues isolated a unicellular cyanobacterium, Cyanothece sp. ATCC51142, on the intertidal sands of the Texas Gulf Coast [62]. Their symbiotic associations play vital roles in chemical defense, as well as supplying partners with energy and organic products of carbon or nitrogen fixation [63]. In numerous oceanic diatom taxa, heterocyst-forming cyanobacterial symbionts are frequently observed [64]. In the microenvironment of a photosynthetic symbiotic partner cell, such as diatoms, heterocyst-forming cyanobacteria have a beneficial effect, where the oxygen-sensitive nitrogenase protein is inhibited as a result of O2 concentrations [10]. Richelia intracellularis J. Schmidt 1901 is a filamentous cyanobacteria frequently found either free-living or in symbiosis with the diatoms Rhizosolenia Brightwell 1858 and Chaetoceros Ehrenberg 1844 in the plankton of the warm oceans [63]. Cyanobacteria nitrogen fixation has various impacts that can be explained as follows: firstly, cyanobacteria are significant bioavailable nitrogen suppliers to the pelagic and benthic food webs that support fish productivity by fixing dissolved N2. The food web benefits from bioavailable nitrogen in addition to the fresh or decaying filamentous cyanobacteria to nourish various invertebrates and release more nitrogen by cyanobacterial cells. Secondly, their ability to get beyond summertime nitrogen limitation by the fixation of dissolved nitrogen. Thirdly, they can contribute to enhancing productivity in different agricultural and ecological situations by building up soil fertility and increasing yield [65][66][67]. The researchers conclude that only filamentous non-heterocyst cases have contributed to the explanation of the mechanism of nitrogen fixation. On the other hand, there are limitations in the data related to unicellular cyanobacteria, and filamentous heterocyst-forming symbionts, as there are no papers explaining the role of the nitrogen fixation mechanism in these cases. Taken together, nitrogen fixation has important impacts in various fields, i.e., agriculture and ecology, reflecting a potential benefit on the economy.
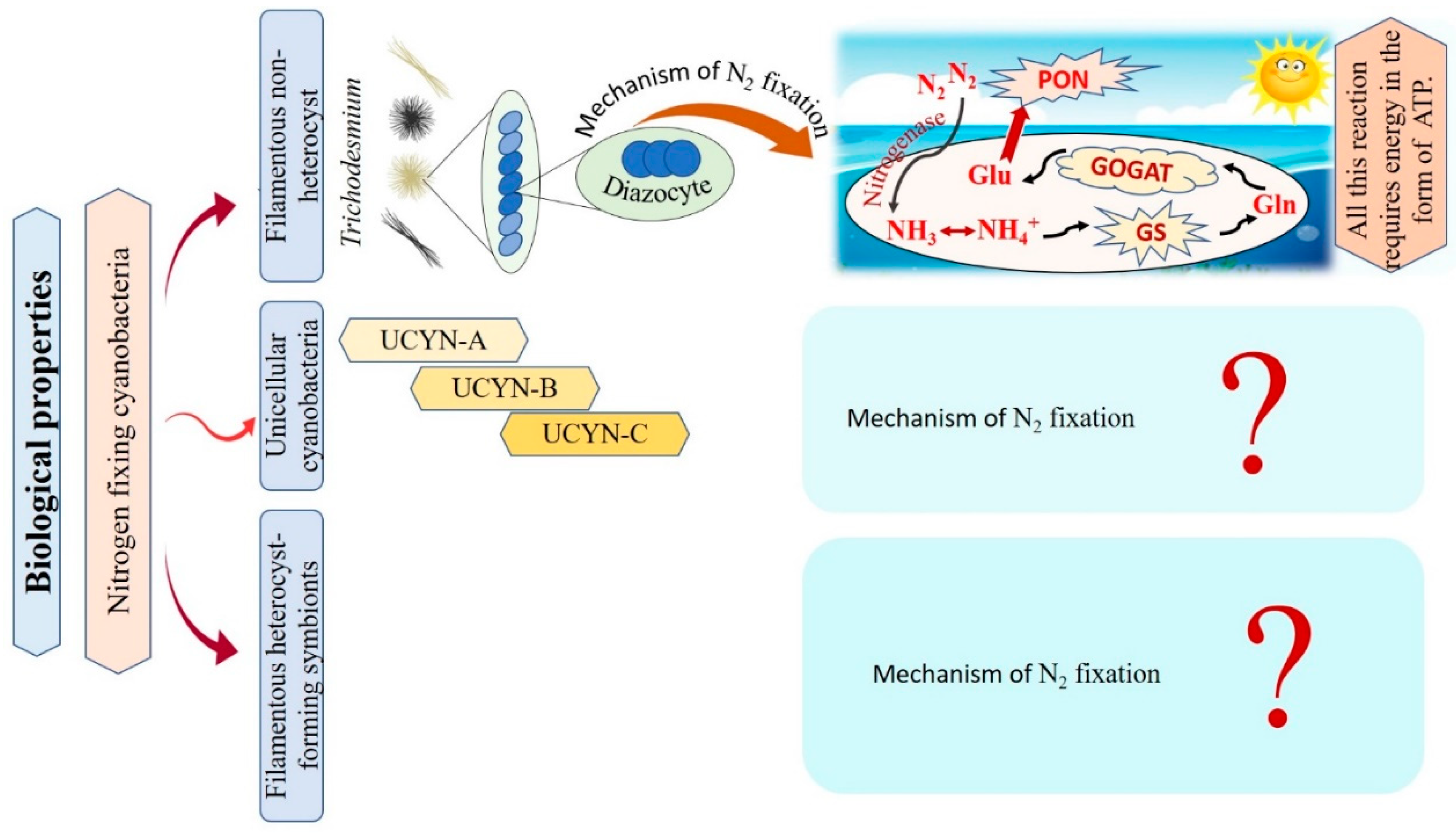
Figure 2. Flowchart for the different types of nitrogen-fixative cyanobacteria with their mechanisms of action.
4. Mechanism of Marine Cyanobacteria in CO2 Fixation
Industrialization and the burning of fossil fuels are to blame for the alarmingly high CO2 levels in the atmosphere. CO2 concentrations in surface waters have increased with the rise in atmospheric CO2 during the past century. Cyanobacteria are one of the most promising organisms for CO2 capture [68][69][70]. The most common photoautotrophic lineage on Earth comprises cyanobacteria. Their effectiveness in photoautotrophism relies on a collection of adaptations known as the CO2-concentrating mechanism (CCM). The CCM aims to increase the efficiency of CO2 fixation by promoting the carboxylase reaction through improving the chemical conditions around the main CO2-fixing enzyme, D-ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO), and suppressing the oxygenase reaction [71]. In cyanobacteria, RubisCO is enclosed within a class of protein-rich structures known as carboxysomes. The selectively permeable protein shell of the carboxysomes contains the CO2-fixation enzyme, as well as the carbonic anhydrase enzyme, which provides CO2 from a cytoplasmic bicarbonate pool [71][72]. The carboxysomes have two main types, α-cyanobacteria, which are found in marine water and β-cyanobacteria, present in fresh water [72]. In α-cyanobacteria, the RubisCO is categorized as (RuBisCO Form IA) [73]. In α-cyanobacteria, up to two different types of plasma membrane-associated bicarbonate transporters, SbtA2 (high-affinity Na+/HCO3−) and BicA2 (medium to low-affinity Na+/HCO3−) promote CO2 fixation [71]. In the first stage of the (CCM), bicarbonate is concentrated inside the cell via transporters in the cell membrane. In the second stage of (CCM), the carboxysome plays a vital role in the enhancement of CO2 fixation by co-localizing the two enzymes (CA) and (RuBisCO). Bicarbonate is assumed to enter the carboxysome through proteinaceous shell pores, and once inside, it is converted to CO2 and used by RuBisCO. The conversion of HCO3− to CO2 is catalyzed by the CA enzyme. After CO2 fixation, some of it exits into the cytosol, while some promptly combines with RuBP to form phosphoglycolate Figure 3 [72][74]. The components of the cyanobacterial CO2-concentrating mechanism (CCM) have a vital role in the improvement of the efficiency of photosynthetic CO2 fixation in the chloroplasts of crop plants via specific cyanobacterial transporters, accordingly leading to better crop yields [75]. Finally, the researchers conclude that the importance of CO2 revolves around the conversion of inorganic carbon to organic carbon through vital processes.
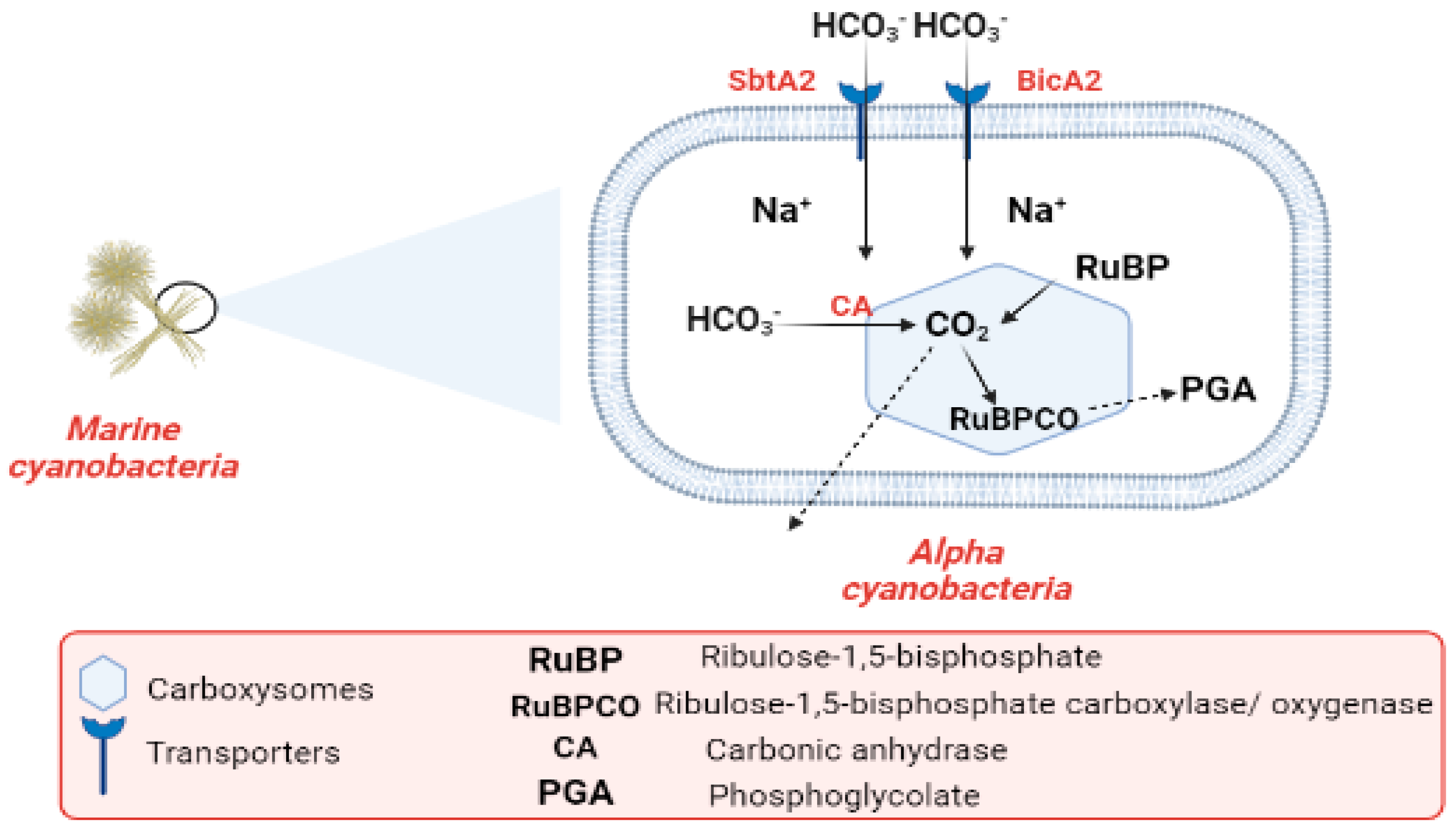
Figure 3. Flow chart explaining the carbon cycle in marine cyanobacteria.
This entry is adapted from the peer-reviewed paper 10.3390/md21080439
References
- Chen, S.; Shi, N.; Huang, M.; Tan, X.; Yan, X.; Wang, A.; Huang, Y.; Ji, R.; Zhou, D.; Zhu, Y.-G.; et al. MoS2 Nanosheets–Cyanobacteria Interaction: Reprogrammed Carbon and Nitrogen Metabolism. ACS Nano 2021, 15, 16344–16356.
- Azeez, R.; Dhanalakshmi, P.K.; Surenirakumar, K.; Nallamuthu, T. Growth and biochemical parameters of selective cultured cyanobacteria and exploiting antibacterial potency against human bacterial pathogens. Elixer. Appl. Bot. 2014, 72, 25537–25543.
- Altermann, W.; Kazmierczak, J.; Oren, A.; Wright, D.T. Cyanobacterial calcification and its rock-building potential during 3.5 billion years of Earth history. Geobiology 2006, 4, 147–166.
- Bishoyi, A.K.; Sahoo, C.R.; Padhy, R.N. Recent progression of cyanobacteria and their pharmaceutical utility: An update. J. Biomol. Struct. Dyn. 2022, 41, 4219–4252.
- Jirí, K.; Kastovský, J. Coincidences of structural and molecular characters in evolutionary lines of cyanobacteria. Algol. Stud. 2003, 109, 305–325.
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353.
- Freeman, C.J.; Thacker, R.W. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol. Oceanogr. 2011, 56, 1577–1586.
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Mar. Drugs 2016, 14, 97.
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315.
- Zehr, J.P. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 2011, 19, 162–173.
- Waterbury, J.B.; Watson, S.W.; Guillard, R.R.L.; Brand, L. Widespread occurrence of a unicellular, marine, planktonic, cyanobacterium. Nature 1979, 277, 293–294.
- Sigman, D.M.; Boyle, E.A. Glacial/interglacial variations in atmospheric carbon dioxide. Nature 2000, 407, 859–869.
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503.
- Ridley, C.P.; Bergquist, P.R.; Harper, M.K.; Faulkner, D.J.; Hooper, J.N.A.; Haygood, M.G. Speciation and Biosynthetic Variation in Four Dictyoceratid Sponges and Their Cyanobacterial Symbiont, Oscillatoria spongeliae. Chem. Biol. 2005, 12, 397–406.
- Donia, M.S.; Hathaway, B.J.; Sudek, S.; Haygood, M.G.; Rosovitz, M.J.; Ravel, J.; Schmidt, E.W. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nat. Chem. Biol. 2006, 2, 729.
- Luesch, H.; Harrigan, G.G.; Goetz, G.; Horgen, F.D. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr. Med. Chem. 2002, 9, 1791–1806.
- Perera, R.M.T.D.; Herath, K.H.I.N.M.; Sanjeewa, K.K.A.; Jayawardena, T.U. Recent Reports on Bioactive Compounds from Marine Cyanobacteria in Relation to Human Health Applications. Life 2023, 13, 1411.
- Kultschar, B.; Llewellyn, C. Secondary Metabolites in Cyanobacteria; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78923-643-9.
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring Marine Cyanobacteria for Lead Compounds of Pharmaceutical Importance. Sci. World J. 2012, 2012, 179782.
- Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P.; Ramaraj, R.; Govindan, N. Exploration of bioactive compounds and antibacterial activity of marine blue-green microalgae (Oscillatoria sp.) isolated from coastal region of west Malaysia. SN Appl. Sci. 2020, 2, 1906.
- Singh, U.; Singh, P.; Singh, A.K.; Laxmi; Kumar, D.; Tilak, R.; Shrivastava, S.K.; Asthana, R.K. Identification of antifungal and antibacterial biomolecules from a cyanobacterium, Arthrospira platensis. Algal Res. 2021, 54, 102215.
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476.
- Demirkiran, O.; Almaliti, J.; Leão, T.; Navarro, G.; Byrum, T.; Valeriote, F.A.; Gerwick, L.; Gerwick, W.H. Portobelamides A and B and Caciqueamide, Cytotoxic Peptidic Natural Products from a Caldora sp. Marine Cyanobacterium. J. Nat. Prod. 2021, 84, 2081–2093.
- Oh, D.-C.; Strangman, W.K.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Thalassospiramides A and B, Immunosuppressive Peptides from the Marine Bacterium Thalassospira sp. Org. Lett. 2007, 9, 1525–1528.
- Gunasekera, S.P.; Kokkaliari, S.; Ratnayake, R.; Sauvage, T.; dos Santos, L.A.H.; Luesch, H.; Paul, V.J. Anti-Inflammatory Dysidazirine Carboxylic Acid from the Marine Cyanobacterium Caldora sp. Collected from the Reefs of Fort Lauderdale, Florida. Molecules 2022, 27, 1717.
- Renugadevi, K.; Valli Nachiyar, C.; Sowmiya, P.; Sunkar, S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp. TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242.
- Gutiérrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a Potent Cytotoxic Cyclodepsipeptide from Papua New Guinea Collections of the Marine Cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103.
- Almaliti, J.; Malloy, K.L.; Glukhov, E.; Spadafora, C.; Gutiérrez, M.; Gerwick, W.H. Dudawalamides A–D, Antiparasitic Cyclic Depsipeptides from the Marine Cyanobacterium Moorea producens. J. Nat. Prod. 2017, 80, 1827–1836.
- Sergeev, V.N.; Gerasimenko, L.M.; Zavarzin, G.A. The Proterozoic History and Present State of Cyanobacteria. Microbiology 2002, 71, 623–637.
- Golubic, S.; Seong-Joo, L. Early cyanobacterial fossil record: Preservation, palaeoenvironments and identification. Eur. J. Phycol. 1999, 34, 339–348.
- Golubic, S.; Hofmann, H.J. Comparison of Holocene and mid-Precambrian Entophysalidaceae (Cyanophyta) in stromatolitic algal mats: Cell division and degradation. J. Paleontol. 1976, 50, 1074–1082.
- Hofmann, H.J. Precambrian Microflora, Belcher Islands, Canada: Significance and Systematics. J. Paleontol. 1976, 50, 1040–1073.
- Lanier, W.P. Interstitial and peloid microfossils from the 2.0 Ga Gunflint Formation: Implications for the paleoecology of the Gunflint Stromatolites. Precambrian Res. 1989, 45, 291–318.
- Sharma, M.; Shukla, Y. The evolution and distribution of life in the Precambrian eon-global perspective and the Indian record. J. Biosci. 2009, 34, 765–776.
- Sharma, M. Palaeobiology of Mesoproterozoic Salkhan Limestone, Semri Group, Rohtas, Bihar, India: Systematics and significance. J. Earth Syst. Sci. 2006, 115, 67–98.
- Sharma, M.; Sergeev, V.N. Genesis of carbonate precipitate patterns and associated microfossils in Mesoproterozoic formations of India and Russia—A comparative study. Precambrian Res. 2004, 134, 317–347.
- Knoll, A.H.; Sergeev, V.N. Taphonomic and evolutionary changes across the Mesoproterozoic-Neoproterozoic transition. Neues Jahrb. Geol. Palaontol. Abh. 1995, 195, 289–302.
- Castenholz, R.W. Species usage, concept, and evolution in the cyanobacteria (blue green algae). J. Phycol. 1992, 28, 737–745.
- Komárek, J. Review of the cyanobacterial genera implying planktic species after recent taxonomic revisions according to polyphasic methods: State as of 2014. Hydrobiologia 2016, 764, 259–270.
- Komárek, J. Recent changes (2008) in cyanobacteria taxonomy based on a combination of molecular background with phenotype and ecological consequences (genus and species concept). Hydrobiologia 2010, 639, 245–259.
- Díez, B.; Bergman, B.; El-Shehawy, R. Marine diazotrophic cyanobacteria: Out of the blue. Plant Biotechnol. 2008, 25, 221–225.
- Capone, D.G.; Burns, J.A.; Montoya, J.P.; Subramaniam, A.; Mahaffey, C.; Gunderson, T.; Michaels, A.F.; Carpenter, E.J. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles 2005, 19, 1–17.
- Stal, L.J. Nitrogen Fixation in Cyanobacteria. eLS 2015, 1–9.
- Breitbarth, E.; Oschlies, A.; LaRoche, J. Physiological constraints on the global distribution of Trichodesmium—Effect of temperature on diazotrophy. Biogeosciences 2007, 4, 53–61.
- El-Shehawy, R.; Lugomela, C.; Ernst, A.; Bergman, B. Diurnal expression of hetR and diazocyte development in the filamentous non-heterocystous cyanobacterium Trichodesmium erythraeum. Microbiology 2003, 149, 1139–1146.
- Wannicke, N.; BP, K.; Voss, M. Release of fixed N2 and C as dissolved compounds by Trichodesmium erythreum and Nodularia spumigena under the influence of high light and high nutrient (P). Aquat. Microb. Ecol. 2009, 57, 175–189.
- Mulholland, M.R.; Capone, D.G. The nitrogen physiology of the marine N2-fixing cyanobacteria Trichodesmium spp. Trends Plant Sci. 2000, 5, 148–153.
- Kranz, S.A.; Eichner, M.; Rost, B. Interactions between CCM and N2 fixation in Trichodesmium. Photosynth. Res. 2011, 109, 73–84.
- Zehr, J.P.; Waterbury, J.B.; Turner, P.J.; Montoya, J.P.; Omoregie, E.; Steward, G.F.; Hansen, A.; Karl, D.M. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature 2001, 412, 635–638.
- Zehr, J.P.; Mellon, M.T.; Zani, S. New Nitrogen-Fixing Microorganisms Detected in Oligotrophic Oceans by Amplification of Nitrogenase (nifH) Genes. Appl. Environ. Microbiol. 1998, 64, 3444–3450.
- Zehr, J.P.; Bench, S.R.; Carter, B.J.; Hewson, I.; Niazi, F.; Shi, T.; Tripp, H.J.; Affourtit, J.P. Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science 2008, 322, 1110–1112.
- Prechtl, J.; Kneip, C.; Lockhart, P.; Wenderoth, K.; Maier, U.-G. Intracellular Spheroid Bodies of Rhopalodia gibba Have Nitrogen-Fixing Apparatus of Cyanobacterial Origin. Mol. Biol. Evol. 2004, 21, 1477–1481.
- Zehr, J.P.; Montoya, J.P.; Jenkins, B.D.; Hewson, I.; Mondragon, E.; Short, C.M.; Church, M.J.; Hansen, A.; Karl, D.M. Experiments linking nitrogenase gene expression to nitrogen fixation in the North Pacific subtropical gyre. Limnol. Oceanogr. 2007, 52, 169–183.
- Stenegren, M.; Caputo, A.; Berg, C.; Bonnet, S.; Foster, R.A. Distribution and drivers of symbiotic and free-living diazotrophic cyanobacteria in the western tropical South Pacific. Biogeosciences 2018, 15, 1559–1578.
- Thompson, A.W.; Zehr, J.P. Cellular interactions: Lessons from the nitrogen-fixing cyanobacteria. J. Phycol. 2013, 49, 1024–1035.
- Sohm, J.A.; Webb, E.A.; Capone, D.G. Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 2011, 9, 499–508.
- Shiozaki, T.; Ijichi, M.; Kodama, T.; Takeda, S.; Furuya, K. Heterotrophic bacteria as major nitrogen fixers in the euphotic zone of the Indian Ocean. Glob. Biogeochem. Cycles 2014, 28, 1096–1110.
- Dron, A.; Rabouille, S.; Claquin, P.; Le Roy, B.; Talec, A.; Sciandra, A. Light–dark (12:12) cycle of carbon and nitrogen metabolism in Crocosphaera watsonii WH8501: Relation to the cell cycle. Environ. Microbiol. 2012, 14, 967–981.
- Compaoré, J.; Stal, L.J. Oxygen and the light–dark cycle of nitrogenase activity in two unicellular cyanobacteria. Environ. Microbiol. 2010, 12, 54–62.
- Turk-Kubo, K.A.; Frank, I.E.; Hogan, M.E.; Desnues, A.; Bonnet, S.; Zehr, J.P. Diazotroph community succession during the VAHINE mesocosm experiment (New Caledonia lagoon). Biogeosciences 2015, 12, 7435–7452.
- Taniuchi, Y.; Chen, Y.L.; Chen, H.-Y.; Tsai, M.-L.; Ohki, K. Isolation and characterization of the unicellular diazotrophic cyanobacterium Group C TW3 from the tropical western Pacific Ocean. Environ. Microbiol. 2012, 14, 641–654.
- Reddy, K.J.; Haskell, J.B.; Sherman, D.M.; Sherman, L.A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 1993, 175, 1284–1292.
- Andreeva, N.A.; Melnikov, V.V.; Snarskaya, D.D. The Role of Cyanobacteria in Marine Ecosystems. Russ. J. Mar. Biol. 2020, 46, 154–165.
- Foster, R.A.; Subramaniam, A.; Zehr, J.P. Distribution and activity of diazotrophs in the Eastern Equatorial Atlantic. Environ. Microbiol. 2009, 11, 741–750.
- Mishra, U.; Pabbi, S. Cyanobacteria: A potential biofertilizer for rice. Resonance 2004, 9, 6–10.
- Karlson, A.M.L.; Duberg, J.; Motwani, N.H.; Hogfors, H.; Klawonn, I.; Ploug, H.; Barthel Svedén, J.; Garbaras, A.; Sundelin, B.; Hajdu, S.; et al. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio 2015, 44, 413–426.
- Pandey, S.N.; Verma, I.; Kumar, M. Chapter 23—Cyanobacteria: Potential Source of Biofertilizer and Synthesizer of Metallic Nanoparticles; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 351–367. ISBN 978-0-12-819311-2.
- Levitan, O.; Rosenberg, G.; Setlik, I.; Setlikova, E.; Grigel, J.; Klepetar, J.; Prasil, O.; Berman-Frank, I. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 2007, 13, 531–538.
- Case, A.E.; Atsumi, S. Cyanobacterial chemical production. J. Biotechnol. 2016, 231, 106–114.
- Zhang, A.; Carroll, A.L.; Atsumi, S. Carbon recycling by cyanobacteria: Improving CO2 fixation through chemical production. FEMS Microbiol. Lett. 2017, 364, fnx165.
- Rae, B.D.; Long, B.M.; Badger, M.R.; Price, G.D. Functions, Compositions, and Evolution of the Two Types of Carboxysomes: Polyhedral Microcompartments That Facilitate CO2 Fixation in Cyanobacteria and Some Proteobacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 357–379.
- Yeates, T.O.; Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C.; Shively, J.M. Protein-based organelles in bacteria: Carboxysomes and related microcompartments. Nat. Rev. Microbiol. 2008, 6, 681–691.
- Turmo, A.; Gonzalez-Esquer, C.R.; Kerfeld, C.A. Carboxysomes: Metabolic modules for CO2 fixation. FEMS Microbiol. Lett. 2017, 364, fnx176.
- Cannon, G.C.; Bradburne, C.E.; Aldrich, H.C.; Baker, S.H.; Heinhorst, S.; Jessup, M. Shively Microcompartments in Prokaryotes: Carboxysomes and Related Polyhedra. Appl. Environ. Microbiol. 2001, 67, 5351–5361.
- Price, G.D.; Pengelly, J.J.L.; Forster, B.; Du, J.; Whitney, S.M.; von Caemmerer, S.; Badger, M.R.; Howitt, S.M.; Evans, J.R. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J. Exp. Bot. 2013, 64, 753–768.
This entry is offline, you can click here to edit this entry!
