Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The over-exploitation of fossil fuels and their negative environmental impacts have attracted the attention of researchers worldwide, and efforts have been made to propose alternatives for the production of sustainable and clean energy. One proposed alternative is the implementation of bioelectrochemical systems (BESs), such as microbial fuel cells (MFCs), which are sustainable and environmentally friendly. MFCs are devices that use bacterial activity to break down organic matter while generating sustainable electricity.
- bioelectrochemical system
- bioenergy
- fuel production
- microbial fuel cell
1. Introduction
In response to the environmental pollution caused by conventional energy sources, researchers worldwide have searched for reliable and sustainable energy production sources. In this line, bioelectrochemical systems (BESs) capable of transforming chemical energy into electrical energy aided by microorganisms as catalysts have been developed [1]. BESs are promising technologies that have been implemented to replace energy sources from fossil fuels, such as petroleum, natural gas, and coal [1][2], in response to worldwide high energy demands. BESs can be classified into (i) microbial desalination cells (MDCs) [3][4][5], (ii) microbial electrosynthesis cells (MECs) [6], (iii) enzymatic biofuel cells (EBCs) [7], (iv) electrolysis cells (ECs) [8], (v) microbial solar cells (MSCs) [9], (vi) biobatteries [10][11], (vii) constructed wetland microbial fuel cells (CW-MFCs) [12], and (viii) microbial fuel cells (MFCs). To date, considering the types of BESs, MFCs are the oldest, being the first BES presented in 1911 [13]. The operation of an MFC is based on bacterial activity, where so-called electrochemically active bacteria (EAB) break down (i.e., oxidize) organic matter (OM) to produce bioelectricity [14]. During this process, the EAB use their metabolic pathway to transport electrons [15][16].
The components of an MFC basically include an anode compartment and a cathode compartment (Figure 1a), both separated internally by a membrane. The anode chamber is mainly responsible for the oxidation of OM (Equation (1)) in the substrate (e.g., in presence of water) through microbial activity, producing electrons (e−), protons (H+), and carbon dioxide (CO2) [17]; then, e− are transported toward the cathode chamber [14][18]. Subsequently, the protons are associated with oxygen, thanks to the PEM (Equation (2)), in order to form an essential compound in the reaction: the water molecule (H2O). Equation (3) indicates the complete chemical reaction in an MFC. Additionally, the electron transfer mechanism at the anode surface of an MFC is presented in Figure 1b. The author referred to the following critical reviews to better understand this area [16][19].
Anode:C6H12O6+6H2O→6CO2+24H++24e−
Cathode:O2+4H++4e−→2H2O
Overall reaction:C6H12O6+6H2O+6O2→6CO2+12H2O
Since the implementation of MFCs, wastewater has been used as an excellent organic substrate for these systems, improving their efficiency in both bioenergy generation and waste management [20][21][22][23][24][25]. The highest power density of 4.99 ± 0.02 W/m2 was reached in a dual-chamber MFC inoculated with an excellent mixed culture [26]. This high performance was obtained in a three-dimensional N-doped bio-anode MFC fabricated with carbon felt as the anode and cathode and was higher than that of previous studies using carbon-based materials. The MFC reactor had a working volume of 80 mL in each chamber. The system’s high performance was likely due to the electrode materials used. More recently, a high power density of 1793 ± 77 mW/m2 was achieved in an MFC operated with atomically dispersed Fe–N4 moieties as an excellent cathode catalyst [27]. The atomically dispersed Fe–N4 moieties were considered a perfect option to enhance the performance of the MFCs.
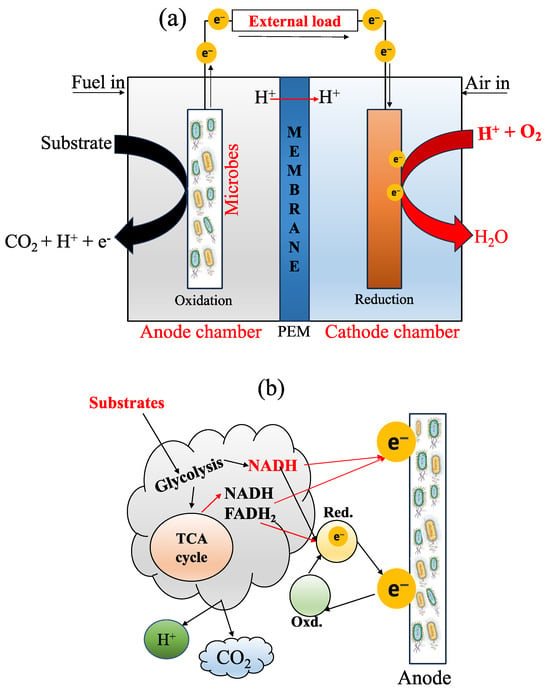
Figure 1. (a) A typical diagram of an MFC with a PEM (proton exchange membrane) and its bioelectrogenic process; and (b) the electron transport mechanism, adapted from [28]. During the process, there are three parameters to consider: the microorganism’s structures to carry out the phenomenon, the type of microorganism, and the electrical conductivity of the anode material. So far, there are three methods for electron transfer through EAB activity: (i) electron transfer through redox-active protein molecules, (ii) the use of shuttle electrons to transfer electrons, and (iii) direct electron transfer through conductive pili [29]. When the material has high conductivity, it helps to improve the flow of electrons, exhibiting less resistance [30]. In addition, the mechanism of electron transfer also involves natural mediators, directors of electron transfer, and synthetic mediators [19].
2. Bioelectricity Production
MFC technologies have received significant attention, due to their extraordinary capacity to produce bioenergy without harming the environment. Figure 2 illustrates the advances that have been made to bring MFC technology into the realm of practical application. To date, more than 7363 investigations have been conducted on the use of MFCs for low-cost electricity generation. In addition, over 3216 review papers have been published on different aspects of MFCs, followed by 2530 book chapters, 388 conference abstracts, and 284 encyclopedias. It is worth mentioning that these data were obtained only from the ScienceDirect database, as there were more publications on energy recovery using MFC systems than on other platforms.
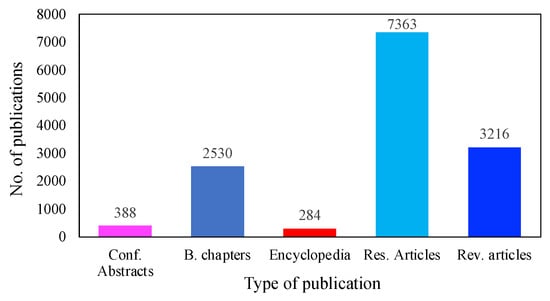
Figure 2. Publications on MFCs during the last decade.
Great strides have been made in improving bioelectricity production by MFCs. Many recent studies have reported high power generation efficiency using MFCs inoculated with different types of waste (e.g., wastewater, sludge, and human urine).
The highest power density of 2203 mW/m2 was obtained using an SC-MFC inoculated with anaerobic sludge as an outstanding inoculum [31]. The SC-MFC was constructed with a graphite brush as an anode, while modified materials—that is, graphite-based nanomaterials and platinum/carbon (Pt/C)—were utilized for the cathode. The performance achieved in the study was referred to as the cathode modification and the operating conditions of the MFC (i.e., 30 days of operation and 27 cycles). In contrast, Subran et al. [32] obtained a highest power density of 590 mW/m2 (with 78% COD removal efficiency) in an SC-MFC using carbon cloth as an anode and cathode, separated by a Nafion 117 membrane. This value was almost four times lower than that reported with Pt/C [31]. In the study by Sathish et al., rGOHI-AcOH (acetic acid) and rGO/Ni (reduced graphite oxide nickel nanoparticle composite)—two reduced graphene oxide hydrogen iodides—were used with Pt/C as catalysts [31]. The results were interesting, as they indicated that the rGOHI-AcOH-based catalysts, as mentioned earlier, could feasibly drive the MFC system without interruption (in terms of power density). The power output found in [32] was 76.6% higher than the maximum power output (138 mW/m2) reported in a tubular MFC (T-MFC) [33]. The T-MFC was fabricated using a graphite rod as an anode electrode, carbon cloth-coated Pt (200 cm2) as a cathode electrode, and nanocomposite as a proton exchange membrane. The findings indicated that the best option for improving the performance of an MFC is modifying its electrode (i.e., synthesized and characterized nanocomposite membranes) and using suitable configuration materials. In the previous study by Bensaida et al. [34], the highest power density of 833.33 mW/m2 was obtained in a prototype MFC inoculated with Mg(OH)2-coated iron nanoparticles. This value was 29.19 and 83.34% superior to that in agro-waste and synthetic wastewater, respectively; however, it was approximately three times inferior to that reported in anaerobic sludge elsewhere [31].
Anaerobic sludge appears to be the most suitable inoculum for use in MFCs to improve the power density efficiency, compared to others. This kind of inoculum (sludge) has tremendous potential to increase the performance of MFC systems [31]. This performance enhancement was due to the proper characteristics related to the sludge used, including operating conditions and modification of the reactor. It is crucial to note that the efficiency of an MFC also depends on factors such as the type of electron acceptors and catalysts used. Both electroactive biofilms and extracellular electron transport in MFCs are highly dependent on the physicochemical properties of the operational anode surface; therefore, surface modification of conventional anode materials is critical for improving the performance of MFCs. However, more studies on the use of substrate and electrode modifications are required in order to reliably increase energy production in MFC technologies.
On the other hand, in their quest to increase the amount of OM and keep bacterial populations active on the anode surface, researchers have investigated other types of substrates with great potential, such as acetate, butyrate, glucose, cellulose, and sucrose. These substrates have all been used in MFCs in an attempt to improve their output power, as described in [2]. The main idea of using such substrates in MFCs is to improve the transport of protons from one compartment to another, in addition to forming good biofilms on the electrode and keeping the bacterial community active. The highest power densities in the range of 305 and 506 mW/m2 have been recorded in an SC-MFC inoculated with butyrate (1000 mg/L) and acetate (800 mg/L), respectively [35]. It was noted that the SC-MFC using acetate presented better performance, in comparison to that inoculated with butyrate. The difference between the reactors regarding the power density arose from the concentrations of the substrates. Later, in an SC-MFC inoculated with glucose, the highest power density of 52 mW/m2 was reported, approximately 9 times and 5 times less than those derived for butyrate and acetate, respectively [36]. Hashmi et al. [37] have recently reported the highest power density of 71.12 mW/m2 in a DC-MFC when removing hazardous materials from wastewater. This yield in MFC was due to the addition of 350 μmol/L of C6N6FeK3 (potassium ferricyanide) in the cathode compartment. Concurrently, 180 μmol/L of CH2 (methylene) was applied to the anode compartment. As mentioned above, a high concentration of the chemical compounds is an excellent option to increase the performance of MFCs while treating wastewater.
According to the above studies, modifying the electrode material is the better or cheaper option to improve MFC performance, in terms of power density, compared to conventional electrodes. The configuration of an MFC using a conventional electrode is expensive, making its large-scale application much more costly. Furthermore, another alternative to improve the performance of MFCs is the use of organic substrates, which are excellent electron donors. Finally, anaerobic sludge has been reported to be one of the best inoculums (due to characteristics such as bacterial diversity, as described earlier) for improving power generation in MFCs, followed by Mg(OH)2-coated iron nanoparticles, rGOHI-AcOH, and acetate as excellent substrates (with excellent carbon sources to improve or stimulate EAB) for MFC technologies [31][35]. Furthermore, algae have been reported to be a good substrate for enhancing the performance of MFCs for the recovery of value-added products from wastewater [38][39], including biofuels [40], bioremediation, and bioelectricity generation [41][42]. According to the literature, MFC-based algae have great potential for improving power efficiency, nutrient removal, heavy metal recovery, and bioremediation of contaminants.
Yaqoob et al. [43] recorded the highest current density of 36.84 mA/m2 (within 20 days of operation) using potato wastewater as an electron donor for the biodegradation of pollutants in a benthic microbial fuel cell (BMFC). This MFC performance was 82.28% less than that previously reported in a DC-MFC operated with potato waste as the substrate [44]. The study by Yaqoob et al. [43] demonstrated the simultaneous effective removal of OM and COD (up to 84%) and bioremediation of pollutants (up to 90%). The electricity generation behavior observed in both studies was due to the configuration materials used to assemble the electrodes. Furthermore, the characteristics of the substrates, such as chemical composition (e.g., pH; electrical conductivity, EC; and so on), as well as the operating conditions, were factors that influenced the performance of the MFCs in both studies. For example, in the study by Yaqoob et al. [45], the pH and EC of the substrate before application were 7.04 (neutral) and 59 μS/cm (increased by 26% after treatment), respectively, which improved the production of power in the BMFC. The neutral pH of a substrate (or environment) facilitates the development or growth of microorganisms, promoting optimal activity levels on the anode surface [46]. In addition, OM concentrations and the bacterial community play critical roles in improving the performance of MFCs.
Furthermore, in another investigation, an SC-MFC was configured using bamboo charcoal (BC) for the anode electrode and a Pt-coated carbon cloth for the cathode [47]. The working volume of the MFCs was 530, 530, and 500 mL, and they were inoculated with potato-processing wastewater. In one MFC, a maximum current density of 1140 mA/m2 was observed. The current density achieved by Sato et al. [47] in potato waste-fed SC-MFC was higher than that in the above studies. This performance could be due to the physicochemical parameters of the substrate (potato waste) and the high EC and low resistance when BC is used as an anode electrode. Other key characteristics of BC include its biocompatibility, high chemical stability, and mechanical strength [47].
In summary, the above findings indicated that potato waste—like other organic substrates—is potentially an excellent alternative, allowing for enhanced power generation and wastewater bioremediation performance when using MFCs. Furthermore, the electrode materials are other aspects to be considered when implementing MFC technology. However, more investigations are required to evaluate the influence of potato wastewater in MFC technological development.
3. Other Types of Energy Production
In recent years, great strides have been made regarding the use of BESs, such as MFCs, for the simultaneous treatment of wastewater and production of fuels. This progress has been achieved by combining BES prototypes to improve their process performance. In this line, the use of MFCs for the production of both CH4 and H2 has captured the attention of researchers worldwide. Apart from the fact that this technology is environmentally friendly, it is also an excellent option for producing fuels while eliminating pollutants in wastewater. Furthermore, MFC technologies are less expensive, when compared to conventional methods.
Hao et al. [48] have recently constructed an SC-MFC (working volume of 2000 mL) coupled with an anaerobic membrane bioreactor (AnMBR) to enable enhanced CH4 production. While 40 cm2 of carbon felt was utilized as an anode, the cathode was constructed from various materials (e.g., non-woven carbon cloth, platinum carbon, and carbon black). Before assembly of the SCMFC-AnMBR, the carbon felt was sterilized using 1 mol/L HCl and NaOH. The SCMFC-AnMBR was inoculated with synthetic wastewater (SWW) and compared to the conventional one (C-AnMBR). The SWW was added to SCMFC-AnMBR with a concentration ratio of 205:5:1 (C:N:P), and the main components of SWW were: (1) 3000 mg/L of glucose (C6H12O6), (2) 120 mg/L of monopotassium phosphate (KH2PO4), (3) 120 mg/L of ammonium chloride (NH4Cl), (4) 112 mg/L of iron chloride (FeCl2), and (5) 30 mg/L of magnesium sulfate (MgSO4). The SCMFC-AnMBR indicated high recovery efficiencies of 38.89 mg/L (COD) and 67.84 mg/L (CH4) and presented a maximum voltage of 107 ± 14 mV.
Additionally, in a previous study by Nguyen et al. [49], single-chamber microbial electrolysis cells (MECs) using a simultaneous dark fermentation (DF) process were constructed. The DF was combined with MECs to form the so-called DF-MEC. Then, the DF-MEC was inoculated with a species of algae called Saccharina japonica (sDFMEC), in order to improve fuel production and accelerate the process. The sDFMEC recorded a maximum production of H2 of 438.7 ± 13.3 mL/g-TS, outperforming the control (i.e., DF-MEC). At the same time, the sDFMEC system reached maximum CH4 production and COD removal of 63.1 ± 3.4 mL/g-TS and 75.6 ± 1.4%, respectively. Nonetheless, DF-MEC showed an efficient recovery of 32.2%, achieving a maximum H2 production of 403.4 mL/g-TS [49]. In contrast, in the study by Gebreslassie et al. [50] a maximum H2 production of 110 mL/g-VS was reported in a similar sDFMEC. This yield was 3.66 times lower than that reported by Nguyen et al. Later, in an sDFMFC constructed using a surface-modified stainless steel mesh cathode, a higher H2 production of 408 mL/g-TS was recorded [51]. The authors modified this system to optimize the electrode performance during the DF process. They revealed that anodization (i.e., coating an oxide layer over a metal to make it corrosion-resistant) is the best alternative for improving fuel production using bioelectrochemical systems. These findings indicate that sDFMEC is more effective than the combination of SCMFC-AnMBR for fuel production, due to the significant effect of adding S. japonica into the sDFMEC reactor. However, all of these methods can feasibly improve CH4 and H2 production while conducting bioremediation of contaminants from wastewater.
Other strategies used to improve fuel production (i.e., H2 and CH4) include the use of other types of substrates, such as biochar, which led to increases in H2 production of up to 118.5% and in CH4 of up to 14% [52]. Calcium lignosulfonate-based biochar led to a 50% increase in H2 [53], a biochar catalyst increased H2 by 52% [54], and electroactive cultures (EACs) have also been tested. More recently, a DC-MFC was constructed to evaluate the effect of CH4 injection on anaerobic oxidation (AO)-coupled MFCs (AO-MFCs), in terms of power generation [55]. Two EACs (acetate and formate) were used in the AO-MFCs, in order to improve the performance of the fabricated prototype. For start-up of the system, 84.5 cm2 (13 × 6.5 cm) of breathable cloth was used as an anode, while the cathode was made of 81 cm2 (9 × 9 cm) of carbon felt, which were separated by an ultrafiltration membrane. The anode chambers were inoculated with 40 mL (0.04 L) of anaerobic sludge. The obtained results indicated that the specific EACs were responsible for converting CH4 to CO2 while producing bioelectricity.
Liu et al. [56] have constructed a novel prototype MEC coupled with AD (MEC-AD; working volume 400 mL) operated with few-layer Ti3C2TX MXene (FL-MXene), multi-layer Ti3C2TX MXene, and MAX phase titanium aluminum carbide (MAX). Subsequently, the reactor was inoculated with cattle manure (CM) and sewage sludge (SS). Furthermore, graphite rods were used for the anode and cathode materials. The MEC-AD operated with a 0.035 wt% of ML-MXene showed a maximum CH4 production of 358.7 mL/g VS. This result was superior to that reported in MECs (38.4 ± 1.7 mL/g TS) elsewhere [49]. The recorded difference was due to the systems having been configured differently and operated under different environmental conditions.
This entry is adapted from the peer-reviewed paper 10.3390/membranes13110884
References
- Sharma, V.; Kundu, P.P. Biocatalysts in microbial fuel cells. Enzyme Microb. Technol. 2010, 47, 179–188.
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003.
- Sadeq, A.M.; Ismail, Z.Z. Sustainable application of tubular photosynthesis microbial desalination cell for simultaneous desalination of seawater for potable water supply associated with sewage treatment and energy recovery. Sci. Total Environ. 2023, 875, 162630.
- Imoro, A.Z.; Mensah, M.; Buamah, R. Developments in the microbial desalination cell technology: A review. Water Energy Nexus 2021, 4, 76–87.
- Jatoi, A.S.; Hashmi, Z.; Mazari, S.A.; Mubarak, N.M.; Karri, R.R.; Ramesh, S.; Rezakazemi, M. A comprehensive review of microbial desalination cells for present and future challenges. Desalination 2022, 535, 115808.
- Tanguay-Rioux, F.; Nwanebu, E.; Thadani, M.; Tartakovsky, B. On-line current control for continuous conversion of CO2 to CH4 in a microbial electrosynthesis cell. Biochem. Eng. J. 2023, 197, 108965.
- Li, Z.; Wu, R.; Chen, K.; Gu, W.; Zhang, Y.H.P.; Zhu, Z. Enzymatic biofuel cell-powered iontophoretic facial mask for enhanced transdermal drug delivery. Biosens. Bioelectron. 2023, 223, 115019.
- Zhang, S.; Yang, C.; Jiang, Y.; Li, P.; Xia, C. A robust fluorine-containing ceramic cathode for direct CO2 electrolysis in solid oxide electrolysis cells. J. Energy Chem. 2023, 77, 300–309.
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263.
- Apollon, W.; Kamaraj, S.K.; Silos-Espino, H.; Perales-Segovia, C.; Valera-Montero, L.L.; Maldonado-Ruelas, V.A.; Vázquez-Gutiérrez, M.A.; Ortiz-Medina, R.A.; Flores-Benítez, S.; Gómez-Leyva, J.F. Impact of Opuntia species plant bio-battery in a semi-arid environment: Demonstration of their applications. Appl. Energy 2020, 279, 115788.
- Apollon, W.; Valera-Montero, L.L.; Perales-Segovia, C.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A.; Gómez-Leyva, J.F.; Vázquez-Gutiérrez, M.A.; Flores-Benítez, S.; Kamaraj, S.K. Effect of ammonium nitrate on novel cactus pear genotypes aided by biobattery in a semi-arid ecosystem. Sustain. Energy Technol. Assess. 2022, 49, 101730.
- Ji, B.; Zhao, Y.; Yang, Y.; Li, Q.; Man, Y.; Dai, Y.; Fu, J.; Wei, T.; Tai, Y.; Zhang, X. Curbing per-and polyfluoroalkyl substances (PFASs): First investigation in a constructed wetland-microbial fuel cell system. Water Res. 2023, 230, 119530.
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. 1911, 84, 260–276.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192.
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723.
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A. Self-sustainable nutrient recovery associated to power generation from livestock’s urine using plant-based bio-batteries. Fuel 2023, 332, 126252.
- Xu, X.; Zhao, Q.; Wu, M.; Ding, J.; Zhang, W. Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe(III) oxide addition. Bioresour. Technol. 2017, 225, 402–408.
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327.
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial fuel cells and their electrified biofilms. Biofilm 2021, 3, 100057.
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307.
- Dai, M.; Wu, Y.; Wang, J.; Lv, Z.; Li, F.; Zhang, Y.; Kong, Q. Constructed wetland-microbial fuel cells enhanced with iron carbon fillers for ciprofloxacin wastewater treatment and power generation. Chemosphere 2022, 305, 135377.
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387.
- Khandaker, S.; Das, S.; Hossain, M.T.; Islam, A.; Miah, M.R.; Awual, M.R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation—A comprehensive review. J. Mol. Liq. 2021, 344, 117795.
- Terbish, N.; Popuri, S.R.; Lee, C.H. Improved performance of organic–inorganic nanocomposite membrane for bioelectricity generation and wastewater treatment in microbial fuel cells. Fuel 2023, 332, 126167.
- Abubackar, H.N.; Biryol, İ.; Ayol, A. Yeast industry wastewater treatment with microbial fuel cells: Effect of electrode materials and reactor configurations. Int. J. Hydrogen Energy 2023, 48, 12424–12432.
- Wang, Y.; He, C.; Li, W.; Zong, W.; Li, Z.; Yuan, L.; Wang, G.; Mu, Y. High power generation in mixed-culture microbial fuel cells with corncob-derived three-dimensional N-doped bioanodes and the impact of N dopant states. J. Chem. Eng. 2020, 399, 125848.
- Wang, X.; Zhang, H.; Ye, J.; Li, B. Atomically dispersed Fe–N4 moieties in porous carbon as efficient cathode catalyst for enhancing the performance in microbial fuel cells. J. Power Sources 2023, 556, 232434.
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A review of recent advances in microbial fuel cells: Preparation, operation, and application. Biotech 2022, 11, 44.
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K. Electrode Material as Anode for Improving the Electrochemical Performance of Microbial Fuel Cells. In Energy Storage Battery Systems—Fundamentals and Applications; Haider, S., Haider, A., Khodaei, M., Chen, L., Eds.; IntechOpen: London, UK, 2021.
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621.
- Sathish, T.; Sathyamurthy, R.; Kumar, S.S.; Huynh, G.B.; Saravanan, R.; Rajasimman, M. Amplifying power generation in microbial fuel cells with cathode catalyst of graphite-based nanomaterials. Int. J. Hydrogen Energy 2022.
- Subran, N.; Ajit, K.; Krishnan, H.; Pachiyappan, S.; Ramaswamy, P. Synthesis and performance of a cathode catalyst derived from areca nut husk in microbial fuel cell. Chemosphere 2023, 312, 137303.
- Sugumar, M.; Dharmalingam, S. Statistical assessment of operational parameters using optimized sulphonated titanium nanotubes incorporated sulphonated polystyrene ethylene butylene polystyrene nanocomposite membrane for efficient electricity generation in microbial fuel cell. Energy 2022, 242, 123000.
- Bensaida, K.; Maamoun, I.; Eljamal, R.; Falyouna, O.; Sugihara, Y.; Eljamal, O. New insight for electricity amplification in microbial fuel cells (MFCs) applying magnesium hydroxide coated iron nanoparticles. Energy Convers. Manag. 2021, 249, 114877.
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662.
- Khater, D.; El-Khatib, K.M.; Hazaa, M.; Hassan, R. Electricity generation using Glucose as substrate in microbial fuel cell. J. Environ. Sci. 2015, 2, 84–98.
- Hashmi, Z.; Jatoi, A.S.; Aziz, S.; Soomro, S.A.; Abbasi, S.A.; Usto, M.A.; Alam, M.S.; Anjum, A.; Iqbal, A.; Usman, M.T. Bio-assisted treatment of hazardous spent wash via microbial fuel cell. Environmental friendly approach. Biomass Convers. Biorefinery 2021, 13, 5981–5989.
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N.L. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041.
- Zhang, Y.; Wang, J.-H.; Zhang, J.-T.; Chi, Z.-Y.; Kong, F.T.; Zhang, Q. The long overlooked microalgal nitrous oxide emission: Characteristics, mechanisms, and influencing factors in microalgae-based wastewater treatment scenarios. Sci. Total Environ. 2023, 856, 159153.
- Shukla, M.; Kumar, S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018, 82, 402–414.
- Mahmoud, R.H.; Samhan, F.A.; Ibrahim, M.K.; Ali, G.H.; Hassan, R.Y. Waste to energy conversion utilizing nanostructured Algal-based microbial fuel cells. Electrochem. Sci. Adv. 2021, 2, e2100071.
- Ribeiro, V.R.; Osório, H.D.D.; Ulrich, A.C.; Rizzetti, T.M.; Barrios, A.S.; de Cassia de Souza Schneider, R.; Benitez, L.B. The use of microalgae-microbial fuel cells in wastewater bioremediation and bioelectricity generation. J. Water Process. Eng. 2022, 48, 102882.
- Yaqoob, A.A.; Al-Zaqri, N.; Yaakop, A.S.; Umar, K. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560.
- Du, H.; Li, F. Enhancement of solid potato waste treatment by microbial fuel cell with mixed feeding of waste activated sludge. J. Clean. Prod. 2017, 143, 336–344.
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable 859 approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928.
- Zhang, L.; Li, C.; Ding, L.; Xu, K.; Ren, H. Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J. Chem. Technol. Biotechnol. 2011, 86, 1226–1232.
- Sato, C.; Paucar, N.E.; Chiu, S.; Mahmud, M.Z.I.M.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes 2021, 9, 2194.
- Hao, Y.; Zhang, X.; Du, Q.; Wang, H.; Ngo, H.H.; Guo, W.; Zhang, Y.; Long, T.; Qi, L. A new integrated single-chamber air-cathode microbial fuel cell-Anaerobic membrane bioreactor system for improving methane production and membrane fouling mitigation. J. Membr. Sci. 2022, 655, 120591.
- Nguyen, P.K.T.; Das, G.; Kim, J.; Yoon, H.H. Hydrogen production from macroalgae by simultaneous dark fermentation and microbial electrolysis cell. Bioresour. Technol. 2020, 315, 123795.
- Gebreslassie, T.R.; Nguyen, P.K.T.; Yoon, H.H.; Kim, J. Co-production of hydrogen and electricity from macroalgae by simultaneous dark fermentation and microbial fuel cell. Bioresour. Technol. 2021, 336, 125269.
- Yun, W.H.; Yoon, Y.S.; Yoon, H.H.; Nguyen, P.K.T.; Hur, J. Hydrogen production from macroalgae by simultaneous dark fermentation and microbial electrolysis cell with surface-modified stainless steel mesh cathode. Int. J. Hydrogen Energy 2021, 46, 39136–39145.
- Li, W.; He, L.; Cheng, C.; Cao, G.; Ren, N. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour. Technol. 2020, 306, 123088.
- Zhao, L.; Zhang, J.; Zhao, W.; Zang, L. Improved Fermentative hydrogen production with the addition of calcium-lignosulfonate-derived biochar. Energy Fuels 2019, 33, 7406–7414.
- Harun, K.; Adhikari, S.; Jahromi, H. Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC Adv. 2020, 10, 40882–40893.
- Zhang, C.; He, P.; Liu, J.; Zhou, X.; Li, X.; Lu, J.; Hou, B. Study on performance and mechanisms of anaerobic oxidation of methane-microbial fuel cells (AOM-MFCs) with acetate-acclimatizing or formate-acclimatizing electroactive culture. Bioelectrochemistry 2023, 151, 108404.
- Liu, J.; Yun, S.; Wang, K.; Liu, L.; An, J.; Ke, T.; Gao, T.; Zhang, X. Enhanced methane production in microbial electrolysis cell coupled anaerobic digestion system with MXene accelerants. Bioresour. Technol. 2023, 380, 129089.
This entry is offline, you can click here to edit this entry!
