Citral is a monoterpene constituted by two isomers known as neral and geranial. It is present in different plant sources and recognized as safe (GRAS) by the Food and Drug Administration (FDA). In recent years, investigations have demonstrated that this compound exhibited several biological activities, such as antibacterial, antifungal, antibiofilm, antiparasitic, antiproliferative, anti-inflammatory, and antioxidant properties, by in vitro and in vivo assays. Additionally, when incorporated into different food matrices, citral can reduce the microbial load of pathogenic microorganisms and extend the shelf life.
- citral
- biological activities
- food additive
- pharmaceuticals
1. Introduction
2. Biological Activities
2.1. Antibacterial Activity
| Microorganism | Dose (MIC) | Effect | Ref. |
|---|---|---|---|
| Bacteria | |||
| V. parahaemolyticus | 0.125 mg/mL | Inhibited bacterial growth, causing damage to bacterial membrane and cell wall. | [47] |
| S. aureus DMST 4745 S. aureus S. agalactiae B. cereus E. coli |
0.62–1.25 μL/mL 0.62–1.25 μL/mL 0.31–0.62 μL/mL 0.15 μL/mL 1.25–2.5 μL/mL |
Citral possessed bacteriostatic and bactericidal actions at different concentrations. | [48] |
| E. coli MG1655 | 300 μL/L | It inactivated at least 2.5 log10 cycles of exponentially growing cells in 3 h under aerobic conditions. | [49] |
| L. monocytogenes S. aureus E. coli |
200 µL/mL 500 µL/mL 500 µL/mL |
Growth inhibition. | [50] |
| L. monocytogenes L. innocua |
0.125 mL/mL 0.125 mL/mL |
Microbial growth of both Listeria species was reduced by almost 2 log10 CFU/mL. | [51] |
| L. innocua L. monocytogenes |
100 µL/mL | Citral in the culture medium of both bacteria provided a reduction of bacitracin from 32 µg/mL to 4 µg/mL, and the colistin changed from 96 and 128 µg/mL for L. monocytogenes and L. innocua, respectively, to 16 µg/mL, for both species. | [52] |
| Salmonella Typhimurium | 3.1 mM | Citral at subinhibitory concentrations (1, 2, and 3 mM) could induce bacterial adaptation and acquire tolerance to inactivation processes. | [53] |
| Fungi | |||
| B. dothidea P. macrospore B. cinerea |
0.2 μL/mL 0.2 μL/mL 0.4 μL/mL |
At 0.4 μL/mL, citral entirely inhibited the growth of all the tested fungi. When concentration reached 0.2 μL/mL, citral inhibited the growth of B. dothidea best, followed by P. macrospore and B. cinerea. | [54] |
| C. sakazakii | 0.8 mg/mL | Growth inhibition and cell damage. | [55] |
| 3600 μM | Concentrations below 225 μM (1/16 MIC) exhibited no inhibition against C. sakazakii ATCC 29544. | ||
| Penicillium roqueforti | 0.17 mg/mL | Citral combination with eugenol damaged the cell membrane, caused a collapse of mitochondria, and inhibited energy production. | [16] |
| Penicillium digitatum | 2.0 or 4.0 μL/mL | Citral altered the mitochondrial morphology, led to the leakage of ATP, and showed an inhibition of the TCA pathway of P. digitatum cells. | [56] |
| S. cerevisiae | 2.0 mM | MIC: Results showed that yeast cells treated with 2 mM citral reached a 95% reduction in CFU/mL. | [57] |
| Zygosaccharomyces rouxii. | 0.188 μL/mL | The minimum fungicidal concentration was 0.375 μL/mL. | [58] |
| Candida albicans | 64 µg/mL | The minimum fungicidal concentration was 256 µg/mL. The MIC and the MFC of citral required only 4 h of exposure to effectively inhibit 99.9% of the inoculum. | [59] |
| Aspegillus niger | 0.23 mg/mL | The combination of citral and eugenol had a synergistic inhibitory effect on A. niger. | [16] |
2.2. Antifungal Activity
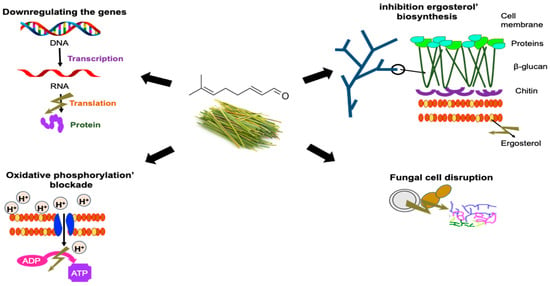
| Microorganism | Mechanism | Ref. |
|---|---|---|
| Saccharomyces cerevisiae BY4741 | Loss of membrane and cell wall integrity results in a typical apoptotic/necrotic cell death. However, yeast cells that escape this first cell membrane disruption, particularly evident in sub-lethal concentration, die by metacaspase-mediated apoptosis induced by the accumulation of intracellular ROS. | [57] |
| B. dothidea | Changes in the morphological characteristics of fungal hyphae, resulting in loss of cell content and distortion of the mycelium. Increase in membrane permeability, with increases in extracellular electrical conductivity and a decrease in soluble protein content. A decrease in the range of ergosterol levels showed that citral altered the physiology of the cell membrane. Reduction in the level of enzymes associated with respiration, resulting in the disruption of energy metabolism. | [54] |
| Aspegillus ochraceus | Citral downregulated ochratoxin biosynthetic genes, including pks and nrps, but slightly upregulated global regulatory factors veA, velB, and laeA. | [54] |
| Aspegillus niger | Direct damage to the cell membranes of A. niger may explain the antimicrobial activity of citral combined with eugenol. Among the two components, eugenol is mainly responsible for the permeability of damaged cell membranes, whereas citral mainly causes membrane lipid peroxidation, which leads to a burst in ROS. | [16] |
| Penicillium roqueforti. |
The combination of citral and eugenol destroyed the integrity of the cell membrane and internal structures and degraded the cell content. The combination induced membrane lipid peroxidation and promoted the ability to destroy the cell membrane. The combined agents eventually caused leakage of cell contents and, ultimately, cell death. | [16] |
| Penicillium digitatum |
Citral can affect the mitochondrial morphology and function of P. digitatum, inhibiting the respiratory metabolism, decreasing the activities of TCA-related enzymes, and changing the TCA metabolic abilities. | [56] |
| Zigosachamomyces rouxii | The antifungal effect can be attributed to the alteration of the integrity and permeability of the cell membrane, which can cause irreversible damage to the cell wall and membrane. They can also destroy yeast proteins and inhibit their synthesis. | [58] |
2.3. Antiproliferative Effect against Cancer Cells
| Compound/ Extract |
Doses | Effect | Ref. |
|---|---|---|---|
| Citral | 145.32 µg/mL 85.47 µg/mL 52.63 µg/mL |
Inhibition of HCT116 cell proliferation (IC50: 24, 48, and 72 h) | [89] |
| Citral | 181.21 µg/mL 143.61 µg/mL 91.5 µg/mL |
Inhibition of HT29 cell proliferation (IC50: 24, 48, and 72 h) | [89] |
| Citral | 3.125–200 µM | Inhibition of CCD841-CoN cell (IC50 not detected at 200 μM) | [89] |
| Citral | 3.7 µg/mL | Inhibition of Caco-2 cell proliferation (IC50: 72 h) | [90] |
| CIT-SNEDDS | 38.50 µg/mL 23.75 µg/mL 16.50 µg/mL |
Inhibition of SW620 cell proliferation (IC50: 24, 48, and 72 h) | [88] |
| CIT-SNEDDS | 44.10 µg/mL 36.60 µg/mL 34.10 µg/mL |
Inhibition of HT29 cell proliferation (IC50: 24, 48, and 72 h) | [88] |
| Citral | 31.25 µg/mL 23.30 µg/mL 22.50 µg/mL |
Inhibition of SW620 cell proliferation (IC50: 24, 48, and 72 h) | [88] |
| Citral | 28.33 µg/mL 22.00 µg/mL 21.77 µg/mL |
Inhibition of HT29 cell proliferation (IC50: 24, 48, and 72 h) | [88] |
| Citral | <25 µg/mL | Inhibition of AGS cell proliferation (IC50: 48 h) | [91] |
| Citral | >75 µg/mL | Inhibition of MRC-5 cell proliferation (IC50: 48 h) | |
| Citral | 1.04 µM | Inhibition of B16F10 cell proliferation (IC50: 24 h) | [92] |
| Citral | 11.7 µM | Inhibition of SK-MEL-147 cell proliferation (IC50: 24 h) | |
| Citral | 13.4 µM | Inhibition of UACC-257 cell proliferation (IC50: 24 h) | |
| Citral | 50.3 µM | Inhibition of HaCaT cell proliferation (IC50: 24 h) | |
| Citral | 2.5 µM | Inhibition of NIH-3T3 cell proliferation (IC50: 72 h) | |
| Citral | 7 µg/mL | Inhibition of HepG2 cell proliferation (IC50: 72 h) | [90] |
| Citral | 1.3 µg/mL | Inhibition of MCF-7 cell proliferation (IC50: 72 h) | |
| Citral | 71.90 µM 57.11 µM 50.20 µM |
Inhibition of KKU-M213 cell proliferation (IC50: 24, 48, and 72 h) | [93] |
| Citral | 94.43 µM 75.06 µM 58.92 µM |
Inhibition of HuCCA-1 cell proliferation (IC50: 24, 48, and 72 h) | [93] |
| Citral | 87.53 72.17 69.22 |
Inhibition of MMNK-1 cell proliferation (IC50: 24, 48, and 72 h) | [93] |
| Citral | 10 µg/mL | Inhibition of PC-3 cell proliferation (IC50: 72 h) | [94] |
| Citral | 12.5 µg/mL | Inhibition of PC-3M cell proliferation (IC50: 72 h) | [94] |
| Citral | >75 µg/mL | Inhibition of MRC-5 cell proliferation (IC50: 72 h) | [94] |
| Citral | 238 µM | Inhibition of PaCa-2 cell proliferation (IC50: 72 h) | [95] |
| Citral | 300 µM | Inhibition of DeFew cell proliferation (IC50: 72 h) | [95] |
| Citral | 5, 10, 20, 40 µg/mL | Inhibit colony formation and migration of AGS (96 h) | [91] |
| Citral | 5, 10, 20, 30, 40 µg/mL | Inhibit colony formation and migration PC-3 (96 h) | [94] |
| Citral | 17.5 and 35 µM | Increase the surviving fraction of KKU-M213 in 106.75 and 115.64% (168 h) | [93] |
| Citral | 23.5 and 47 µM | Decrease the surviving fraction of HU-CCA-1 in 76.35 and 57.71% (168 h) | [93] |
| Citral | 24 and 48 µM | Decrease the surviving fraction of MMNK-1 in 98.46 and 85.26% (168 h) | [93] |
| Citral | 0.25, 0.375, 0.50 mM 0.25, 0.375, 0.50 mM |
Decrease the clonogenicity of HaCaT in 0.3, 4, and 7% (3 h) Decrease the clonogenicity of HaCaT in 22, 28, and 30% (8 h) |
[96] |
| Citral | Decrease the clonogenicity of M624 in 20, 38, and 50% (3 h) | [96] | |
| Citral | 50 µM 100 µM 200 µM |
Early apoptosis (17.1%), late apoptosis (3.1%) in HCT116 (24 h) Early apoptosis (14.2%), late apoptosis (15.1%) in HCT116 (24 h) Early apoptosis (26.2%), late apoptosis (25.8%) in HCT116 (24 h) |
[89] |
| Citral | 50 µM 100 µM 200 µM |
Early apoptosis (22.3%), late apoptosis (16.1%) in HCT116 (48 h) Early apoptosis (26.2%), late apoptosis (24.6%) in HCT116 (48 h) Early apoptosis (32.1%), late apoptosis (37.5%) in HCT116 (48 h) |
[89] |
| Citral | 50 µM 100 µM 200 µM |
Early apoptosis (6.5%), late apoptosis (3.9%) in HT29 (24 h) Early apoptosis (8.5%), late apoptosis (14.2%) in HT29 (24 h) Early apoptosis (8.4%), late apoptosis (24.9%) in HT29 (24 h) |
[89] |
| Citral | 50 µM 100 µM 200 µM |
Early apoptosis (14.5%), late apoptosis (7.1%) in HT29 (48 h) Early apoptosis (22.7%), late apoptosis (17.8%) in HT29 (48 h) Early apoptosis (30.5%), late apoptosis (23.5%) in HT29 (48 h) |
[89] |
| Citral | 10 and 20 µg/mL | Induce early and late apoptosis in AGS | [91] |
| Citral | 1 µM | Apoptosis induction by annexin V-FITC/PI staining in B16F10 (24 h) |
[92] |
| Citral | 0.5, 1, and 2 µM | Apoptosis induction by TUNEL assay in B16F10 (24 h) | [92] |
| Citral | 10 µg/mL 20 µg/mL |
Early apoptosis (44.1%), late apoptosis (52.6%) in PC-3 (48 h) Early apoptosis (62.2%), late apoptosis (38.4%) in PC-3 (48 h) |
[94] |
| Citral | 50, 100, and 200 µM | Disruption of MMP (19.5, 38.8 and 60.9%) in HCT116 (24 h) | [89] |
| Citral | 50, 100, and 200 µM | Disruption of MMP (34.9, 56.4 and 77.3%) in HCT116 (48 h) | [89] |
| Citral | 50, 100, and 200 µM | Disruption of MMP (20.4, 28.2 and 41.9%) in HT29 (24 h) | [89] |
| Citral | 50, 100, and 200 µM | Disruption of MMP (24.5, 43.9 and 59.9%) in HT29 (24 h) | [89] |
| Citral | 50, 100, and 200 µM | Increase intracellular ROS level (1.26, 2.07, and 3.19 folds) in HCT116 (4 h) | [89] |
| Citral | 50, 100, and 200 µM | Increase intracellular ROS level (1.21, 1.39, and 2.25 folds) in HC29 (4 h) | [89] |
| Citral | 50, 100, and 200 µM | Decrease intracellular GSH level in HCT116 (4 h) | [89] |
| Citral | 50, 100, and 200 µM | Decrease intracellular GSH level in HT29 (4 h) | [89] |
| Citral | 1 µM | Autophagic vacuole induction formation in B16F10 (24 h) | [89] |
| Citral | 0.5, 1, and 2 µM | DNA damage in B16F10 (24 h) | [92] |
| Citral | 2.5 µM | Reduction of malondialdehyde level in B16F10 (24 h) | [92] |
| Citral | 10 and 20 µg/mL | Inhibition of lipid droplet accumulation in PC-3 (48 h) | [94] |
| Citral | 50, 100, and 200 µM | Down-expression of Bcl-2 and Bcl-xL proteins in HCT116 (24 h) High expression of Bax, p53, and caspase-3 proteins in HCT116 (24 h) |
[89] |
| Citral | 50, 100, and 200 µM | Down-expression of Bcl-2 and Bcl-xL proteins in HT29 (24 h) High expression of Bax, p53, and caspase-3 proteins in HT29 (24 h) |
[89] |
| Citral | 0.5 and 1 µM | Down-expression of ERK1/2, PI3K, AkT in HCT116 (24 h) High expression of p53 in HCT116 (24 h) |
[92] |
| Citral | 1 µM | Increase cytoplasmatic NF-κB in B16F10 (24 h) | [92] |
| Citral | 1 µM | Decrease nuclear translocation of NF-κB in B16F10 (24 h) | [92] |
| Citral | 0.25, 0.375, 0.5 mM | Caspase-3 activation in M624 (3 h) | [96] |
| Citral | 0.25, 0.375, 0.5 mM | Caspase-3 activation in HaCaT (3 h) | [96] |
| Citral | 20 µg/mL | Down-expression of HMGR, SREPB1, and ACC proteins in PC-3 (48 h) Up-expression of AMPαK in PC-3 (48 h) |
[96] |
| Citral | 5, 10, and 20 µg/mL | Down-expression of BCl-2 in PC-3 (48 h) and high expression of BAX proteins in PC-3 (48 h) |
[96] |
| Citral | Not reported | mRNA upregulate in AGS (48 h): MAPK, Nf-κB, PI3K-Akt, p53, and other signaling pathways. Spliceosoma, apoptosis, and prostate cancer, among others. | [91] |
| Not reported | mRNA downregulate in AGS: NF-κB, PI3K-Akt, p53, PPAR, among other signaling pathways. Cell cycle, fatty acid metabolism, and proteoglycans in cancer, among others. | [91] | |
| Citral | 5, 10, and 20 µg/mL | Down-expression of HMCR, ACC, FASN, and SREPB1 mRNAs in PC-3 (48 h) | [94] |
2.4. Anti-Inflammatory
| Citral/EO Citral Rich/Constituent | Concentration | Animal/Cell Line Tested | Results | Ref. |
|---|---|---|---|---|
| Citral | 5–100 μg/well | Peritoneal macrophage of male BALB/c mice | 50 and 100 μg of citral significantly inhibited IL-1β and IL-10 release and LPS activation. IL-6 production by macrophages significantly decreased at citral concentrations of 5, 10, 25, 50, and 100 μg/well). |
[99] |
| Citral | 0.36, 0.15, and 0.06 g/kg |
MRSA-infected mice | Citral significantly reduced the levels of TNF-α, IL-6, IL-1β, malondialdehyde, and hydroxyl radicals. Increased superoxide dismutase and glutathione enzyme levels. Reduced the lung inflammatory infiltrates infected by MRSA. |
[100] |
| Citral | 300 mg/kg | Diabetes-induced rats | Gene expression of IL-6 and TNF-α in the liver were significantly downregulated. | [101] |
| Citral | 50–300 mg/kg | Paw edema-induced mice | Reversed paw edema formation in mice induced by LPS and zymosan, inducers of TLR4 and TLR2 signaling. | [42] |
| Citral | 300 mg/kg | Eutrophic and obese mice | Citral reduced TNF-α and serum leptin concentration after the LPS challenge. IL6 levels in the hypothalamus of obese mice were reduced. |
[102] |
| Citral | 125, 250, and 500 mg/kg | Male Swiss mice | Citral reduced NO production and inhibited neutrophil migration in liver. | [103] |
| Citral | 10, 20, and 40 mg/kg 3, 6, and 12 µg/mL |
Mice with LPS-induced acute lung injury Alveolar macrophages |
On in vivo LPS-induced acute lung injury, citral reduced TNF-α, IL-6, and IL-1β production. In vitro, citral inhibited the production of TNF-α, IL-6, and IL-1β in alveolar macrophages. The mechanism was associated with PPAR-γ activation. |
[104] |
| Citral, neral, and geranial | 66 µM | Murine J774A.1 macrophages | Citral inhibited TNF-α and IL-6. Pure neral inhibited TNF-α secretion by 60–80%, whereas geranial 57–75%. Both neral and geranial reduced IL-6 secretion of LPS-stimulated macrophages and the expression of inflammatory mediators IL-1β, iNOS, COX-2, and NLRP-3. |
[105] |
| Citral-rich fractions of Citrus lemon EO | 0.005, 0.01, and 0.02% | Murine macrophage RAW264.7 cell line | Reduced the expression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in LPS-induced macrophages. | [106] |
| Cymbopogon citratus EO | 0.1% | Pre-inflamed human dermal fibroblasts | Significantly inhibited the production of the inflammatory biomarkers: vascular cell adhesion molecule 1 (VCAM-1), interferon gamma-induced protein 10 (IP-10), interferon-inducible T-cell alpha chemoattractant (I-TAC), and monokine induced by gamma interferon (MIG). | [107] |
| Myrcia ovata EO | 200 and 300 mg/kg | Male Swiss mice with induced acute inflammation | Reduced leukocyte extravasation and inhibited TNF-α production by 50% and 69% at both concentrations, as well as IL-1β production by 47%. | [108] |
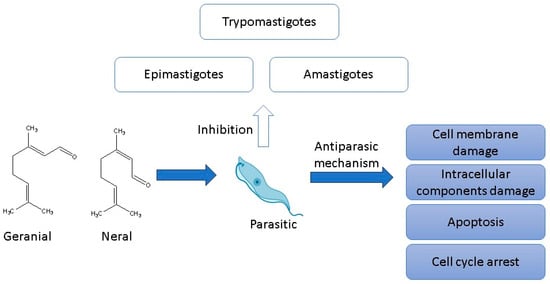
2.5. Antiparasitic
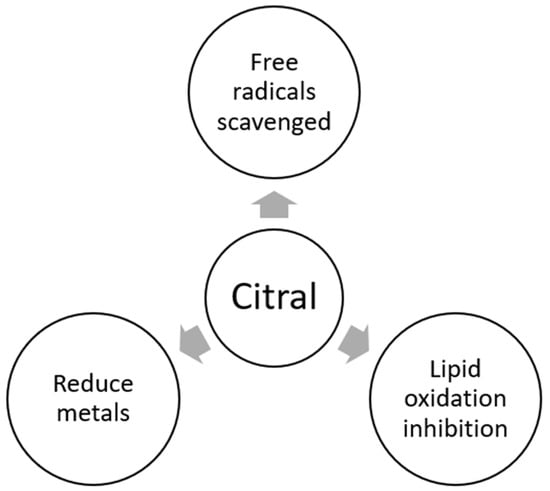
2.6. Antioxidant
3. Use as Possible Food Additive and Pharmaceutical
Citral’s inclusion in the US Environmental Protection Agency (EPA)’s GRAS list as a biopesticide has made it a versatile natural preservative for various food products. This not only extends shelf life but also aligns with consumer demand for healthier, eco-friendly, and clean-label options. hen et al. [129] reported that liposome–citral nanoencapsulates (105.7–238.0 nm) significantly improved the quality of fresh Shatangju mandarins compared to free citral-treated samples by reducing their weight loss and microbial spoilage after storage at 25 °C and 60–70% relative humidity (RH) for 26 d. Nanoemulsions containing citral have also shown outstanding effects when incorporated into coatings as vehicles, such as the case of the study reported by Machado [130], where alginate-based coatings that include citral nanoemulsions, in an optimal concentration between 0.1–0.5%, were a good barrier against microbial attack, while the quality parameters of the fruit were positively affected (e.g., color and respiration rate) during storage for 12 d at 4 °C and 90% RH.
Furthermore, citral’s antifungal activity has been potentiated when combined with other EOs. For example, a nanoemulsion blending clove (CO) and lemongrass (LGO) oils as eugenol and citral sources effectively disrupted the membrane of the highly invasive Fusarium oxysporum f.sp. lycopersici fungus. When tested individually, this combination exhibited the lowest MIC value at 3.9 mg/L, compared to 31.3 for CO and 62.5 mg/L for LGO [139]. Similarly, combining citral and eugenol at concentrations of 60 and 170 mg/L resulted in more substantial damage to P. roqueforti than when these compounds were used separately, leading to the cell content destruction and, consequently, the death of the fungus [16]. The combination of EOs proves to be a cost-effective strategy, achieving superior results with lower component concentrations. Furthermore, developing nanosystems, such as nanoemulsions and nanoencapsulates, facilitates their incorporation into food products, ensuring prolonged bioactivity.
In vitro and in vivo studies have shown that citral is a potent agent with many biological activities. However, citral’s ADME-Tox properties (absorption, distribution, metabolism, excretion, and toxicity) have been poorly understood. An in silico study showed that citral isomers (cis-citral and trans-citral) have acceptable drug-likeness properties and do not present any violations of Lipinski’s rules (molecular weight <500 daltons, LogPo/w value <5, <5 hydrogen bond donors, <10 hydrogen bond acceptors) which guarantees their high absorption when administered orally, however, their low solubility in water limits its distribution. Another important factor to consider is that the plasmatic concentration of citral isomers can be reduced due to the high capacity of both compounds to bind to plasmatic proteins [94]. On the other hand, citral isomers could present a high plasma half-life (T1/2) because both compounds do not show an inhibitory effect on CYP2D6. Citral isomers have an acceptable partition coefficient (cis-citral Log Po/w = 2.74, trans-citral Log Po/w = 2.71), suggesting both compounds can enter the cell and recognize their therapeutic targets. In addition, the predictive carcinogenicity effect in rodents is variable for the citral isomers (cis-citral toxicity (R) = negative, trans-citral toxicity (R) = positive); therefore, it is important to consider the concentration of individual isomers during the preclinical evaluations [9].
These results suggest that citral isomers have an acceptable capacity to be absorbed through the gastrointestinal tract and enter the target cells. However, several research studies do not consider the low distribution and bioavailability of this compound, so it is necessary to focus on the design of formulations that guarantee the compound´s good distribution and bioavailability.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12111608