Cardiac resynchronization therapy (CRT) is a cornerstone therapeutic opportunity for selected patients with heart failure. For optimal patient selection, no other method has been proven to be more effective than the 12-lead ECG, and hence ECG characteristics are extensively researched. The evaluation of particular ECG signs before the implantation may improve selection and, consequently, clinical outcomes. The definition of a true left bundle branch block (LBBB) seems to be the best starting point with which to select patients for CRT. Although there are no universally accepted definitions of LBBB, using the classical LBBB criteria, some ECG parameters are associated with CRT response. In patients with non-true LBBB or non-LBBB, further ECG predictors of response and non-response could be analyzed, such as QRS fractionation, signs of residual left bundle branch conduction, S-waves in V6, intrinsicoid deflection, or non-invasive estimates of Q-LV which are described in newer publications.
1. Introduction
Biventricular pacing significantly decreases QRS duration and consequently improves cardiac performance [
1]. Several major randomized studies [
2,
3,
4,
5,
6,
7]—with the widening of the QRS as the main inclusion criteria—have shown that CRT is beneficial in patients with heart failure with reduced systolic function. Alongside pharmacologic treatment, it is proven to be an effective tool to reverse ventricular remodeling and reduce the rate of hospitalization and improve survival.
While the clinical indication for CRT has in essence remained stable, the criteria relating to ECG parameters have changed over time. Previously, it was mainly the QRS duration that determined which—otherwise optimally managed, but still symptomatic—patients with persistently impaired left ventricular systolic function should receive CRT. Previous guidelines [
8,
9] have determined this value as ≥120 ms. In case of a narrow QRS, CRT was not only proven to be less useful, but it seemed to be disadvantageous as it increased mortality even if left ventricular dyssynchrony was evident by echocardiography [
10,
11,
12]. Meanwhile, accumulating evidence showed that in one third of the patients receiving CRT, no improvement in symptoms and/or in left ventricular ejection fraction could be observed [
13,
14].
Additional data also showed that for patients with symptomatic heart failure, the wider the QRS is, the greater the benefit can be obtained from CRT with regard to hospitalization and mortality [
3]. Furthermore, a meta-analysis of randomized trials drew attention to the finding that patients with moderate QRS prolongation (i.e., 120–149 ms) might not benefit from CRT with respect to adverse clinical events, in contrast to patients having a QRS duration of more than 150 ms [
15].
Beyond the matter of QRS duration, morphological criteria have also been added to the guidelines. Patients with a left bundle branch block (LBBB) pattern demonstrated more benefit than those with right bundle branch block (RBBB) or non-specific intraventricular conduction delay (IVCD) patterns [
16,
17,
18]. Patients with the combination of a normal PR interval and non-LBBB morphology may even have a higher mortality risk after CRT [
19]. Currently, only symptomatic patients with LBBB and a QRS duration of at least 150 ms in their native ECG have a class IA indication for CRT. Recommendations are weaker for patients with an LBBB pattern and a QRS duration of >130 ms and patients with a non-LBBB pattern and a QRS duration of >150 ms, and questionable for patients with a non-LBBB pattern and a QRS duration of 130–149 ms [
20,
21].
2. The problem of Defining the “True” Left Bundle Branch Block
LBBB is defined differently by the WHO, in the American and European guidelines, and also in major clinical CRT studies, like MADIT-CRT and REVERSE [18,21,22,23,24]. Patients with wide QRS who do not meet left bundle branch block definitions and who are thus classified as non-LBBB patients receive different recommendations for CRT. If strict criteria are applied during patient selection, potential responder patients may be deprived of receiving CRT. On the other hand, if less strict criteria are used, patients who may not have any benefit may receive CRT and incur unwanted consequences, such as costs or possible complications.
According to theoretical considerations, CRT is less useful if the failure of the intraventricular electrical impulse propagation is distal to the specialized conducting system and the delay is in the ‘myocyte to myocyte’ phase. However, the actual site of the block in the left bundle cannot be determined with certainty based on the surface 12-lead ECG.
Strauss et al. [
23] pointed out that 30% of the patients diagnosed with LBBB actually have the combination of left anterior fascicular block and left ventricular hypertrophy. For this reason, they suggested raising the conventional QRS duration limit of 130 ms. This is because in a real LBBB it takes at least 40 ms for the impulse to get from the right ventricular endocardium to the left ventricular endocardium. From there on, an additional 50 ms is required to reach the posterolateral wall, and an additional 50 ms to radially spread and activate the posterolateral wall. Regarding the individual (e.g., gender) differences in ventricular wall thickness, this altogether lasts for 130–140 ms at the lowest estimate in adults (
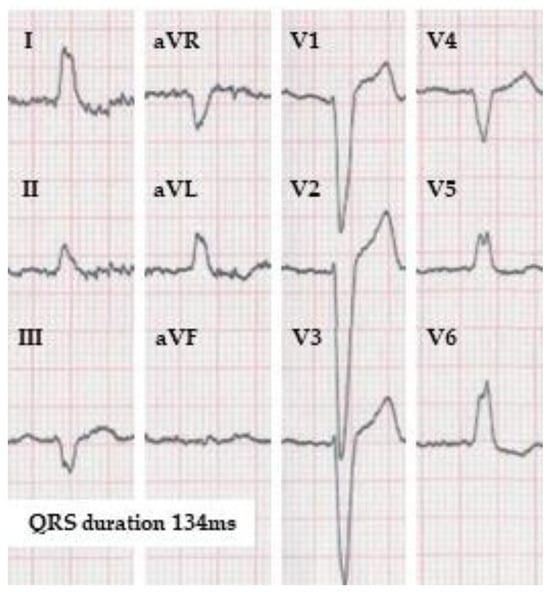
Figure 1).
Figure 1. ECG of a patient fulfilling the strict left bundle branch block definition according to Strauss et al. in case it belongs to a female, but not to a male. The QRS duration is 134 ms (by the automatic measurement). Strauss et al. propose QRS duration limit for strict LBBB as 130 ms for women and 140 ms for men. (10 mm/mV, 25 mm/s).
Patients having a real complete LBBB after suffering a myocardial infarct, may subsequently have a pathological Q-wave in the lateral leads or a high amplitude R-wave evolving septally. According to some LBBB definitions, these patients should be classified into the nonspecific intraventricular conduction disturbance group, though the conduction disturbance of the left bundle would remain.
The uncertainty related to the definition of LBBB is well demonstrated in the study of van Stipdonk et al., where the preprocedural ECGs of 1492 patients who underwent CRT implantation were analyzed [
28]. They found that only 13.8% of the patients could be classified as LBBB by all of the LBBB definitions used in the American and European guidelines, in the MADIT-CRT study, and according to Strauss et al. Moreover, they have found that from the eleven individual ECG characteristics of the different LBBB definitions, only three were independently associated with clinical outcomes. These signs were QS or rS in lead V1; notching or slurring in lead V5, V6, I or aVL; and the absence of a Q wave in leads V5, V6, I and aVL.
It is notable that, whichever definition was used for a study, LBBB was always associated with a better outcome in resynchronization therapy when compared to non-LBBB. Furthermore, most of the studies found that patients who had “true LBBB”, according to the stricter definition of LBBB, had a greater improvement in left ventricular function compared to other patients selected for CRT [
29,
30,
31,
32].
3. ECG Signs Predicting CRT Response beyond the Classical LBBB Markers
3.1. QRS Fractionation
Significant effort has been made to estimate the extent of myocardial fibrosis or scarring and their association with CRT response. The short-duration fractionation caused by left ventricular dyssynchrony is typically located at the mid-QRS. More than two notches of the R wave or in the nadir of the S wave is unexpected in left ventricular dyssynchrony. The more leads with fragmented QRS, the worse the response was to CRT [
33]. In patients with LBBB and a non-ischemic etiology of heart failure, a shorter time (<32.5 ms) from the QRS onset to the beginning of the QRS fragmentation, and a longer fractionation duration, also predicted CRT non-response [
34].
3.2. Signs of Residual Left-Bundle Branch Conduction
The q waves in lateral leads can indicate either a residual LBB conduction or apical myocardial infarction or cardiac fibrosis, each resulting in a significantly lower rate of CRT response in patients with LBBB [
37]. Similarly, an R wave in V1 ≥1 mm may either signify a residual LBB conduction, or a previous posterior wall infarction. The absence of an R wave in V1 (≥1 mm) also predicts a greater response to CRT [
32].
3.3. S-Waves
An S wave in V6 has a high predictive value to identify patients with complete LBBB who may have poor CRT response [
38]. The reason for the presence of an S wave in V6 in complete LBBB is not yet unequivocally explained. A simultaneous delay in the activation of the right ventricle (for example because of a right ventricular pressure overload and enlargement), or posterosuperiorly pointing to late left ventricular activation because of biventricular enlargement [
39], or an apical location of a branch of the left posterior fascicle [
40], are suspected. Poposka et al. [
41] also found that a larger R/S amplitude ratio in V6 is a predictor of CRT response in both LBBB and non-LBBB patients.
3.4. Axis Deviation
The presence of a left or right axis deviation showed controversial results in different publications; therefore, a clear conclusion regarding the association between CRT response and axis deviation cannot be drawn [
43,
44,
45]. Nonetheless, patients with left axis deviation tended to have a shorter left ventricular activation delay, a more extensive apical and anteroseptal scar volume, and more pronounced hypertrophy, reflecting an underlying more advanced structural disease compared to patients with a normal axis.
3.5. Universal QRS Signs of Delayed Left Ventricular Activation
With the help of some newly recognized parameters of the 12-lead ECG, a significant portion of patients could be identified as having a delayed left ventricular activation but categorized as non-LBBB according to the strict LBBB criteria; consequently, they may be excluded from CRT.
The intrinsicoid deflection (ID), which is the time from the earliest onset of the QRS to the point where the maximum deflection towards the baseline starts, represents the time when the electrical activation reaches the cardiac area beneath a particular lead. It seems reasonable that the prolongation of the ID in lateral leads predicts response to CRT.
Vereckei et al. created two formulas to estimate the left ventricular intra- and interventricular dyssynchrony [
47]. The intraventricular dyssynchrony is characterized by the difference of the ID times of the lateral wall (aVL) and the inferior wall (aVF) divided by the total QRS duration: [aVLID − aVFID]/QRSd (%). Interventricular dyssynchrony is calculated as the difference of the intrinsicoid deflection of the left (V5) and right (V1) ventricle divided by the total QRS duration [V5ID − V1ID]/QRSd (%). According to their findings, a value greater than 25% in either case was predictive for CRT response, predominantly in the IVCD group.
The Q-LV interval is the time measured from the onset of the QRS (representing the activation of the septum) on the 12-lead ECG, to the first large peak of the left ventricular electrogram obtained from the left ventricular pacing lead. This is invasively acquired data, which helps to select the latest activating ventricular region from the potentially reachable pacing sites. A longer Q-LV interval is strongly associated with better clinical response [
48,
49]. The comparison of Q-LV with non-invasively obtainable 12-lead ECG parameters revealed that mid-QRS notching and/or slurring, even in one lateral lead, a QRS duration greater than 150 ms, and an ID longer than 60 ms in V6, are associated with a prolonged Q-LV in patients who did not meet the “true LBBB” criteria proposed by Strauss et al. In their study, they also found that patients with atypical RBBB (RBBB morphology in the chest leads, but with the absence of a wide negative terminal deflection in the lateral limb leads) had a long (>110 ms) Q-LV as well [
50]. This kind of “atypical RBBB” that resembles masquerading bundle branch block [
51]—first described by Richman and Wolff in 1954 [
52]—is well known to be associated with poor prognosis [
53] (
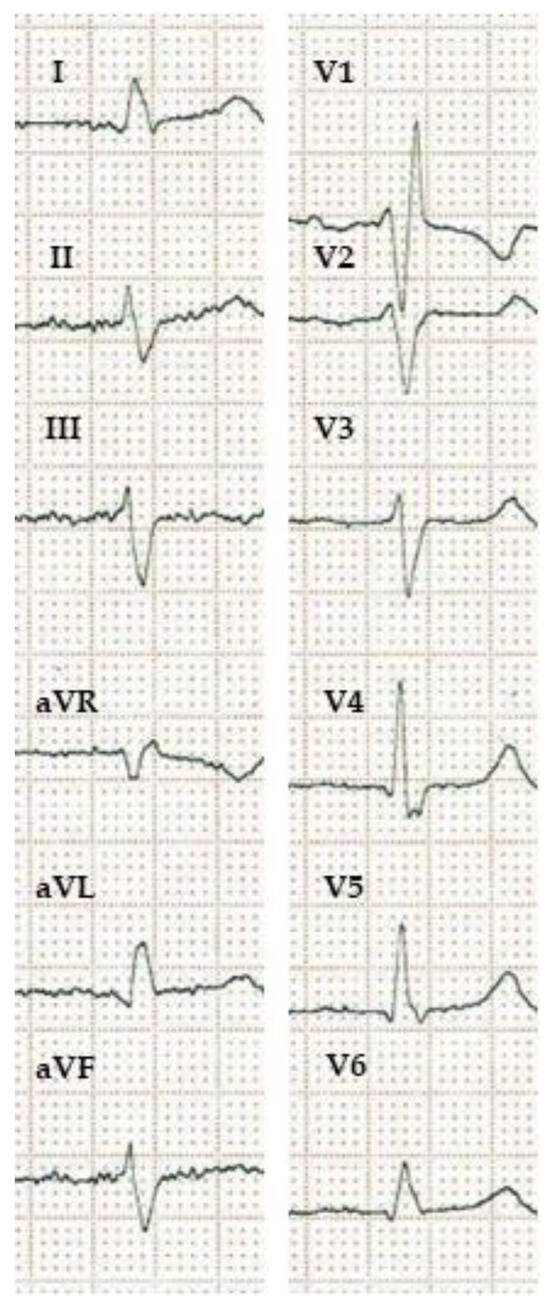
Figure 2).
Figure 2. ECG showing masquerading bundle branch block. Masquerading bundle branch block refers to an intraventricular conduction disturbance signified by a right bundle branch block pattern in the precordial leads with the absence of a wide negative terminal deflection in the lateral limb and/or chest leads and a left bundle branch block-like pattern in the lateral leads. It indicates both right and left ventricular activation delay and usually poor prognosis. Patients with masquerading bundle branch block may have a good response to CRT. (10 mm/mV, 25 mm/s).
3.6. The Prolongation of the PR Interval
The literature regarding the association between a baseline PR interval and CRT response is controversial. In general, patients with a prolonged PR interval are known to have worse outcomes compared to those with a normal PR [
58]. Kutyifa et al. have shown that patients with non-LBBB and a prolonged PR had better outcomes than patients with non-LBBB and a normal PR [
19].
4. Conclusions
Until now, no other modality than electrocardiography has been proven to be better in predicting the clinical response to CRT. The definition of a true LBBB seems to be the best starting point with which to select patients for CRT. From the classical LBBB criteria, the following three seem to be the most important ones: QS or rS in lead V1; notching or slurring in lead V5, V6, I or aVL; and absence of a q-wave in leads V5, V6, I and aVL. It should also be noted that when stricter definitions of LBBB are used, the better predictions for CRT response is expected.
In patients with non-true LBBB or non-LBBB, further ECG predictors of response and non-response could be analyzed, like QRS fractionation, signs of residual left-bundle branch conduction, S-waves in V6, intrinsicoid deflection, or non-invasive estimates of Q-LV; these predictors might also add some information beyond the classical LBBB markers for predicting CRT response. However, important limitations should be acknowledged. Signs that can be read from the 12-lead ECG can be hard to memorize due to their complexity and diverseness, and their reproducible perceptivity is influenced by technical and human factors. Moreover, the lack of universal definitions has an impact on the applicability of even simple criteria of current CRT guidelines.
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10100425