1. Introduction
The neurological effects have been defined in the context of the following spectrum of biological events that appeared to be approached by kratom:
The central nervous system actions of kratom’s alkaloids and their derivatives are the subject of growing scientific interest. Two ways that mitragynine’s effects on the nervous system were revealed in 1932 were effects on the autonomic nervous system, which included facilitation of impulse passage affecting both the cranio-sacral and sympathetic divisions, and another effect on the central nervous system, which included excitation of the medulla, likely the motor centers [
108]. Kratom was found to show an anti-depressant activity at the behavioral level [
27]. Mitragynine, the major compound of kratom, was found to activate the GABAB receptor in a mitragynine-induced conditioned place preference test in rats [
109]. Mitragynine was also found to show a weak functional AMPA and NMDA receptor antagonist action [
110]. However, the neuroendocrine hypothalamic–pituitary–adrenal axis is overactive, as evidenced by the excess production of monoamine neurotransmitters including serotonin, noradrenaline, and dopamine. Nonetheless, the complex pharmacological profile of raw kratom extracts may be explained by the Mitragyna alkaloids’ apparent diverse activities at other brain receptors, such as adrenergic, serotonergic, and dopaminergic receptors [
111]. Chronic mitragynine (5–15 mg/kg; i.p.) injection for 28 days before a working memory test in mice markedly decreased locomotor activity in an open-field test and object identification [
28]. Acute oral administration of kratom extract had no discernible effects on mice’s short-term memory or their ability to coordinate their movements when tested with the rota-rod and the Y-maze, but it did increase their exploratory activity in the Y-maze [
112]. A human study published in 2018 found that frequent kratom users’ motor, memory, attention, and executive function were unaffected by consuming more than three glasses of kratom juice per day [
36]. Chronic morphine, Δ-9-tetrahydrocannabinol, or kratom administration impaired spatial learning and memory processing [
113]. The methanolic extract of kratom (100–1000 mg/kg) was reported to promote learning by demonstrating the latency as a deficiency in memory consolidation of a passive avoidance test. In a two-way active avoidance task, the methanolic extract of kratom had no discernible effects on long-term memory consolidation. The methanolic extract inhibited long-term potentiation (LTP) induction but promoted short-term potentiation in hippocampal field excitatory postsynaptic potentials (fEPSP), demonstrating the impact of extract constituents on the brain’s learning and memory pathways [
114].
2. Kratom, an Indole-like Alkaloid for Neurological Effects
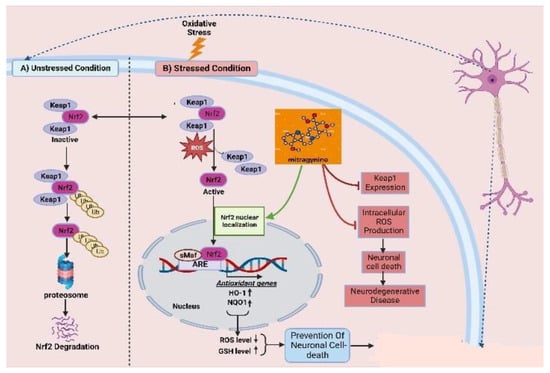
As one of the most abused plant psychotic drug sources, kratom possesses a powerful psychoactive compound in the form of mitragynine, which demonstrates opioid-like behavioral effects and results in neuroplasticity in the reward system of the brain. There are evident and reported cognitive impairments associated with its chronic administration. By combining increased efficacy with better tolerability and a sparing of opioids, multimodal analgesic strategies are paving the way for major improvements in the management of pain. The association of analgesics with different mechanisms of action has proven to be a successful strategy for the treatment of a wide range of pain conditions, minimizing side effects and maximizing the advantage of additive and synergistic effects of the individual agents. Mitragynine, the most common and concentrated indole alkaloid of kratom, is postulated to be involved in the regulation of the Keap-1/Nrf-2 pathway to ensure neuroprotection. The mechanism by which mitragynine exerts its complex effects adrenergic, serotonergic, and opioid-like activity is structurally and pharmacologically distinct from that of traditional opioids. In addition to mitragynine and many of this category’s alkaloids, many of them may play a pivotal role in regulating the overproduction of intracellular ROS, which contribute to neuronal cell death via H
2O
2 exposure. It has been shown that the activation of antioxidative genes of Nrf2, such as HO-1 and NQO1, is primarily dependent upon the nuclear translocation of Nrf2 (
Figure 1) [
115]. This implies that Nrf2 translocation from the cell cytosol to the nucleus plays an important role, while indole-like alkaloids (e.g., prenylated alkaloids) inhibit Keap1, resulting in Nrf2 nuclear translocation. By activating Nrf2, HO-1 and NQO1 are expressed, resulting in a decreased level of ROS and an increased level of GSH, thereby protecting neurons from oxidative damage.
Figure 1. Keap1–Nrf2-linked neuroprotective effects of kratoms’ major compound mitragynine. Oxidative stress leads to dissociation of the Keap1–Nrf2 conjugation, homeostatic condition, and releases the Nrf2. The resulting Nrf2 becomes translocated to the nucleus and upregulates the expression of antioxidative genes. Mitragynine is proposed to facilitate the translocation of Nrf2 to the nucleus, while Keap1 expression seems responsible for neuronal cell death and other neurodegenerative diseases.
3. Anti-Inflammatory Effects Leading to Neuroprotective Effects
In a recent study, kratom has been shown to inhibit proinflammatory mediators release, reduce vascular permeability, and enhance immunity [
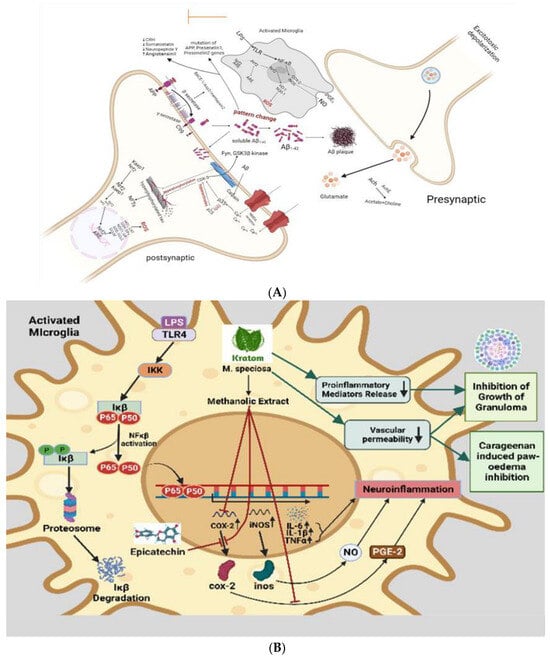
107]. Activated inflammatory cells at the site of infection release inflammatory mediators like cytokines, arachidonic acid, and chemokines, which in turn trigger signal transduction cascades and changes in transcription factors like nuclear factor kappa B (NF-κB), signal transducer and activator of transcription 3, activator protein-1, NF-E2-related factor-2, nuclear factor of activated T cells, and hypoxia-inducible factor-1
α (HIF1-
α). initiation of cyclooxygenase-2 (COX-2) (
Figure 2A), inducibility of nitric oxide synthase (iNOS), and high expression of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and chemokines (CXC chemokine receptor 4) [
116,
117]. Epicatechin has effective anti-inflammatory effects. One of the most potent inflammatory mediators is PGE2. Prostaglandin PGE2 is produced by COX-1 and COX-2, which are cyclooxygenases involved in the inflammatory pathway. Previous research demonstrates that the methanolic extract of kratom inhibits COX-2 mRNA and protein expression in RAW264.7 macrophage cells, as well as PGE2 production [
118]. The Aβ plaque comprises Aβ peptides derived from APP through enzymatic cleavage via (α, β, and γ) secretases. Aβ1-42 readily aggregates and forms the plaque that activates calpain and deregulates p35 into p25, which hyperactivates CDK5 and leads to hyperphosphorylation of tau and the formation of NFTs. β amyloid plaques promote neurotoxicity or activation of microglia by upregulating NF-κB and AP-1 transcription factors, which in turn release ROS and pro-inflammatory cytokines like NO, PGE2, IL-1, IL-6, COX-2, and TNF-α that damage cholinergic neuron (
Figure 2B). These pro-inflammatory cytokines also directly stimulate astrocytes, which create their cytokines to increase inflammatory signals, leading to neuroinflammation and neurodegeneration. Daily intraperitoneal injection of 100–200 mg/kg kratom methanolic extract also had a significant inhibitory effect on the development of granuloma tissue, as demonstrated by the proliferation of modified macrophages, fibroblasts, and highly vascularized, reddish mass tissue, as well as a significant inhibitory effect on the progression of carrageenan-induced paw oedema. In spite of some question marks, inflammation response mechanism, inflammatory cytokines, activation of the inflammasome, and metabolic syndrome as a cause of inflammation in neurotoxicity show that anti-inflammation and neuroprotection are strongly correlated [
119]. In the study, the authors suggested that kratom’s anti-inflammatory properties could be attributed to its inhibition of the release of proinflammatory mediators and its effect on vascular permeability, as well as enhanced immunity and stimulation of tissue repair and healing processes [
21].
Figure 2. (A) Speculated neuroprotective mechanism of kratom showed that inhibit COX-2 activity. In the neuroinflammation pathway, microglial cells are activated by LPS. As a result of oxidative stress, and inflammatory cytokines, some proteins are phosphorylated and activated and result in the phosphorylation and ubiquitination of NF-κB. Then, NF-κB is secreted to the nucleus where it can bind to a specific binding site to activate the transcription and translation of inflammatory cytokines (TNF-α and IL1-β) and proteins (COX-1, COX-2, and iNOS), which are released from the microglia. Kratom inhibits COX-2 activity. (B) Anti-inflammatory action-based neuroprotective effect of kratom. Inflammatory status in activated microglia is initiated through LPS-mediated TLR, IKK, Ikβ, and NFkβ activation leading to the synthesis of interleukin and TNFα which are thought to be neuroinflammatory modulators. While kratom and its alkaloids are postulated to inhibit COX-2, proinflammatory mediators and a decrease vascular permeability imply the contribution of kratom in neuroprotection.
4. Analgesic and Anti-Nociceptive Effects
The first case of using kratom was as an anesthetic by a patient with chronic pain [
120]. In both a concentration-dependent and a time-dependent manner, indole alkaloids such as mitragynine and other derivatives (7-HMG, SC, PAY, and SG) isolated from kratom inhibit electrically induced contractions. Naloxone reversed the opioid receptor agonistic action of electrical stimulation on the guinea pig ileum using the switch contraction of the ileum, which was measured by the opioid receptor agonist action of electrical stimulation [
78,
121,
122]. There was a delay in nociceptive responses to noxious stimulation by both methanolic and alkaloid kratom leave extracts in mice in the hot-plate test, but not in the tail-flick test [
123]. Furthermore, a methanolic extract of kratom was shown to possess antinociceptive activity when it significantly reduced writhing responses and pain sensations in a study where acetic acid was used as the writhing stimulus and the formalin test was administered [
21]. Comparing the antinociceptive effects of various oral kratom extracts with morphine in rats, researchers concluded that alkaloids (20 mg/kg), methanolic extracts (200 mg/kg), and aqueous extracts (100–400 mg/kg) all prolonged the latency of nociceptive responses in both the hot plate and the tail flick-tests. Administrating naloxone before the administration of morphine blocks its effects, suggesting that the opioid receptor may play a partial role in mediating those effects [
124]. In comparison to 5 mg/kg morphine, these effects were less pronounced, but they were more evident than after 100 mg/kg paracetamol [
125]. The anti-nociceptive activity of the alkaloid extract of kratom was potentiated by co-administration of caffeine (25 mg/kg, p.o.) and codeine (3 mg/kg, p.o.) in a hot plate test in rats [
90,
126]. 7-HMG exhibits more potent antinociceptive activity in the tail-flick and hot-plate tests than morphine when administered subcutaneously or orally. The ability of 7-HMG to penetrate the blood–brain barrier (BBB) and exert a more rapid effect than morphine has been attributed to its higher potency and rapid effect [
68,
122]. 7-HMG was also confirmed to have a high level of potency in opioid receptors. The analgesic properties of mitragynine are 13 times greater than those of 7-HMG, while 3–4 times higher for mitragynine [
23,
127]. There was also evidence that 7-HMG, a minor constituent of kratom, was 46 times more potent as an analgesic than mitragynine in another study [
122]. In humans, it exerts sedative and analgesic effects at higher doses and stimulant effects at low doses [
38]. Due to this characteristic of the plant, drug addicts have been highly tempted to abuse it [
128]. In the tail-flick test in mice, intracerebroventricular administration of mitragynine and mitragynine pseudoindoxyl had an antinociceptive effect with an ED
50 estimate of 60.22 nM and 6.51 nM, respectively. The antinociceptive effects of mitragynine and mitragynine pseudoindoxyl were blocked by naloxone, indicating that they are mediated by opioid receptors [
78,
121]. By blocking 1-opioid receptors, the antinociceptive effect of 7-HMG was eliminated in both tail-flick and hot-plate tests since its antinociceptive action is dose-dependent and predominantly mediated through these receptors [
129]. It has been shown that mitragynine binds strongly to the μ-opioid receptors and has analgesic, respiratory depression, and euphoric effects [
130,
131]. A part of the antinociceptive activity of 7-HMG has also been attributed to the supraspinal-μ and δ-opioid receptors [
6,
130,
131]. In addition to alleviating withdrawal symptoms, kratom can be used to diminish the effects of opium addiction. However, it has a lower affinity for the κ-receptor [
131]. Through presynaptic dopamine actions, the κ-receptor exhibited analgesic and depressive effects on locomotor activity [
132]. 7-HMG’s supportive actions are partially mediated by μ and δ-opiate receptors [
133]. In contrast, a study revealed that kratom powder has less affinity for the μ-opioid receptor than morphine [
134]. In mice, the head-twitch reaction brought on by activating postsynaptic 2-adrenoceptors can be reduced by mitragynine and the 5-HT2A receptor antagonist ritanserine. Mitragynine and 7-hydroxymitragynine may produce antinociceptive synergism with adrenergic-α2 (Aα2R) and μ-opioid receptor agonists, according to Obeng, S [
135]. Mitragynine may potentially generate hypothermic synergism when paired with Aα2R agonists. The improvement in positive and negative psychotic symptoms by the methanolic extract of kratom may be attributable to the inhibition of D
2 and 5-HT
2 receptors [
23,
136]. Mitragynine may inhibit NG108-15 cell adenylyl cyclase via opioid receptors. Mitragynine can limit neurotransmitter release by reversibly inhibiting neuronal Ca
2+ channels, which may lead to a reduction in neurotransmitters and an inhibition of pain transduction [
122]. In the heart, kratom inhibits hERG-mediated K+ currents and prolongs action duration, constituting a major risk of cardiotoxicity due to blockage of the human Ether-a-go-go-Related Gene (hERG) channel [
137].
5. Neurological Effects by Gene Regulation
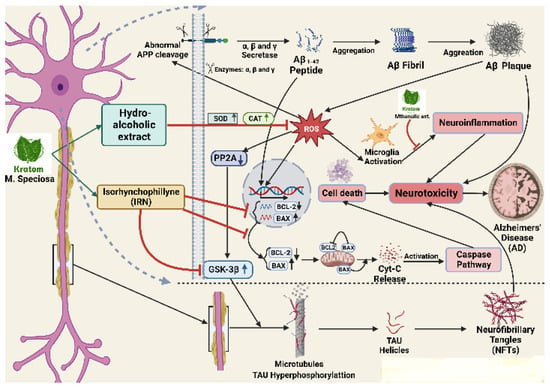
Figure 3 has been summarized to show the gene cascade for neuroprotection by kratom. An interesting finding has been those two alkaloids, bufanidrine (2) and buphanisine (3), have a high affinity for the serotonin reuptake transport protein (SERT). The neuroprotective effects of the Amaryllidaceae alkaloids were considered to be associated with the 1,3-dioxole moiety in those alkaloids, which was thought to be responsible for their neuroprotective effects in AD [
138,
139,
140]. According to previous studies on MAO inhibitory effects of some plant alkaloids, bitter leaf alkaloid-rich extract (BLAE) might produce these effects as a result of its constituent alkaloids [
141,
142]. Several amine neurotransmitters, such as noradrenaline, dopamine, and serotonin, are oxidized by the MAO enzyme, which is a strategic neuronal enzyme [
141]. Furthermore, elevated MAO activity has been directly linked to AD and Parkinson’s disease (PD) because of excessive enzymatic depletion of neuroactive amines and the generation of free radicals which initiate and propagate oxidative stress in AD brains [
141,
143,
144,
145]. As a result, inhibiting MAO activity is a valuable restorative methodology for AD and PD [
146].
Figure 3. Cascade of gene regulation by kratom to control neuroinflammation. Abnormal APP cleavage eventually assists the formation of Aβ plaque which is stimulated by ROS and increases the neuroinflammation in many ways including BCL-2 decrease, BAX increase, microglia activation, and PP2A activation leading to GSK-3B activation. The activation of GSK-3B finally increases NFTS formation. Kratom’s alcoholic extract inhibits ROS production and its products (IRN) inhibit GSK-3B to substantially inhibit the NFTs formation.
As a potential treatment for AD, it has been reported that repression of acetylcholine esterase (AChE), butylcholine esterase (BChE), ATPase, ADPase, and MAO activity may be effective in combination. The enzymes superoxide dismutase (SOD) and catalase (CAT) are also important antioxidant enzymes that serve to prevent the toxicity of hydrogen peroxide (H
2O
2). They were observed to be markedly increased in the brain tissue with the treatment of kratom hydroalcoholic extract in an animal model [
147]. It has been discovered that elevated amounts of Aβ oligomers promote the development of oxidative stress, neuroinflammation, synapse loss, and nerve cell death. In another current study, mitragynine was shown to inhibit the enzyme acetylcholinesterase (AChE) involved in AD [
148]. α-synuclein expression was established to increase in PD and several alkaloids, such as physostigmine, that are reported to attenuate the expression of the α-synuclein gene. ROS, which is also the primary cause of the aberrant aggregation of Aβ peptides that causes the progression of AD which is particularly sensitive in the brain [
149]. The alkaloid Isorhynchophylline is beneficial for treating AD because of its neuroprotective properties through lowering the levels of Bcl-2/Bax gene expression by reducing Aβ-tempted neuronal apoptosis of neurons in the hippocampus. In the Aβ-transgenic CL2006 and CL4176 strains, palmatine, a naturally occurring isoquinoline alkaloid, greatly reduced Aβ-induced paralysis and showed neuroprotective benefits. A variety of antioxidant defense mechanisms, which include the involvement of antioxidant enzymes, like SOD and CAT, mediate the removal of excessive ROS. In wild-type nematodes, palmatine increased the expression of heat shock genes (
shsp), such as
hsp-16.11,
hsp-16.2, and
hsp-16.49, and it increased the intensity of
hsp-16.2p: GFP fluorescence in transgenic CL2070 nematodes. As a result, it is probable that sHSP’s enhanced expression reduced protein aggregation and reduced Aβ toxicity, indicating that sHSP is crucial to Palmatine’s neuroprotective benefits. Heat shock factor (HSF-1) is a transcription factor that is known to have a major role in regulating the production of sHSP. Its decreasing activity has been linked to several detrimental processes that occur in neurodegenerative diseases [
150,
151]. The results are from previous studies that suggest HSF-1 is involved in the inhibition of Aβ toxicity [
151,
152]. As a result, the regulator HSF-1, and modulation of the expression of its target genes, including
hsp-16.11,
hsp-16.2, and
hsp-16.49, are involved in palmatine-mediated suppression of Aβ toxicity. In comparison to morphine, speciociliatine, and mitragynine had DNA protection capacities that were, respectively 1200- and 20- fold higher. In a dose-dependent manner, mitragynine, the main component of the alkaloid extracted from kratom, was administered at concentrations ranging from 0.5 to 20 g/mL. This resulted in a significant inhibition of the mRNA expression of COX-2 induced by LPS, which was followed by a decrease in PGE 2 production, implying mitragynine’s anti-inflammatory effects [
118]. The contribution of all these alkaloids is hypothesized to impact the neuroprotective effect of alkaloids of alkaloid-rich plant products kratom either in a direct or in a cascade mechanism of neuroprotection.
6. Antioxidative Effects
By limiting the start or growth of oxidative chain reactions, antioxidants are those substances that can delay or hinder the oxidation of lipid or auxiliary molecules [
153]. They can extend shelf life and neutralize free radicals by postponing the oxidation of lipids. Antioxidants are available in both natural and synthetic forms. While synthetic antioxidants are produced using synthetic chemicals, natural antioxidants are obtained by extracting natural substances that can snare free radicals [
154]. However, numerous types of research on the potential of naturally occurring antioxidants produced from plants have been conducted due to worries about the adverse effects of utilizing synthetic antioxidants. These can prevent degenerative illnesses and food-based fat oxidation [
155]. Kratom exhibits antioxidant action, which has primarily been attributed to the presence of polyphenolic substances such as flavonoids and alkaloids (
Table 1). DPPH free radical scavenging, lipid protection of DNA, and prevention of metal-induced protein oxidation against H
2O
2-induced oxidative stress were all used to assess antioxidant capabilities [
156]. According to reports, the polar nature of the antioxidant biomolecules may be seen in the fact that aqueous and methanol extracts from several of plant sources have better free radical scavenging action than dichloromethane or ethyl acetate extracts [
154]. By using several techniques, including the 2,2-diphenyl-1-picrylhydrazyl (DPPH), reducing power, oxygen radical absorption capacity test (ORAC), FRAP, and CUPRAC procedures, the relevance of antioxidant activity in kratom was revealed [
157]. Additionally, 100 mg/kg of the kratom aqueous extract significantly increased the specific activity of glutathione-S transferase (GSTs) by 129% compared to the control [
89]. Another study showed the ethanolic extract of kratom exposed antioxidant effects on DPPH, and phytochemical screening [
16,
18]. Kratom was already reported to inhibit the release of proinflammatory mediators, especially NF-κB, interleukins, and cytokines. And antioxidants exert a regulatory effect on the expression of pro-inflammatory cytokines [
158].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28217372