The richest sources of vitamin C are fruits from different parts of the world, such as the kakadu plum from Australia, camu-camu and acerola from South America, and rosehip and sea buckthorn in Europe and Asia. Star fruit, guava, black currant, kiwifruit, and strawberry are good sources of dietary AA; citrus fruits have a lower but acceptable content of vitamin C. AA is also contained in cruciferous vegetables, kale, peppers, spinach and other green-leaf vegetables, tomatoes, asparagus, and Brussels sprouts. A significant amount of AA can also be found in fresh aromatic herbs and algae, while grains, starchy roots and tubers, and animal products, i.e., meat, eggs, and dairy, contain very little AA [
10,
12,
13]. In addition to AA from fresh vegetables and fruits, extraction of AA allows the reuse and valorization of some waste materials produced by the food industry. Every year, it generates a massive amount of waste material, such as peels and seeds, from juice and jam production, causing environmental and economic problems. However, this waste is still a rich source of bioactive compounds, allowing the combination of these valuable compounds’ extraction with the recycling of waste materials from the food industry [
14,
15,
16,
17]. For instance, orange is one of the most well-known and rich sources of AA and, alongside the pulp, the peel contains three times more vitamin C than the inner, edible part of the fruit [
18,
19]; pomegranate peel is a rich source of tannins, flavonoids, and other phenolic compounds, and its extract, with antioxidant and antimutagenic properties, contains more AA than the fruit or the seeds [
20,
21].
2. AA as Dietary Supplement
Vitamin C plays a critical role as an antioxidant and a cofactor in enzyme reactions, involved in the maintenance of healthy body structure in the skin, bones, teeth, tendons, and blood vessels; it is also involved in iron adsorption and immune response regulation [
22]. Animals can synthesize this vitamin in the liver or kidneys. In contrast, humans and primates have lost this ability due to a mutation in the coding sequence of the enzyme L-gulono-gamma-lactone oxidase. Thus, its intake is derived from the diet [
23,
24]. The recommended daily dose is still widely debated, with variations in guidelines depending on country, region, and organization, ranging between 40 and 220 mg. This recommended intake aims to maintain the level of plasmatic AA at 50–75 μmol/L; if it decreases to under 23 μmol/L, the affected organism experiences hypovitaminosis C, with related symptoms such as fatigue, lethargy, and mood changes. If it further decreases to under 11 μmol/L, there is a vitamin C deficiency [
25,
26]. Besides the different guidelines’ values, recommendations regarding sufficient intake are more or less the same, and fall into four categories: preventing scurvy, saturating immune cells with AA whilst limiting its urinary excretion, maintaining an adequate plasma concentration of AA by replacing its daily turnover, and optimizing individual health [
27]. The levels of plasmatic AA vary with age and gender; it is higher in children of 6 to 11 years old, then it decreases over time, and after 60 years old, it increases again. Regarding gender, it is higher in women than men [
28].
AA concentrations are affected by various factors, i.e., dietary intake, absorption, distribution, oxidative decomposition, and excretion. Oral ingestion is the first route of administration of vitamin C, and in humans, it is usually stored in the skeletal muscles; healthy subjects can saturate the level of muscle AA using only a balanced diet, while diseased individuals and smokers may need supplements [
29].
Vitamin C can be administered using different routes. The oral route is strictly ruled by intestinal adsorption and limited by an active transport mechanism; the intravenous route avoids the gastrointestinal passage, increasing the plasma levels until renal clearance re-establishes homeostasis. For some more peripheral applications, a topical route is preferred, to improve bioavailability [
30].
In general, oral administration is the most commonly used method; thus, its homeostasis is driven by the interplay of different mechanisms: intestinal absorption by sodium-dependent vitamin C transporter 1 (SVCT1); kidney filtration and reabsorption in the proximal tubule through SVCT1; cells’ internalization mediated by sodium-dependent vitamin C transporter 2 (SVCT2); and urinary excretion [
12,
31].
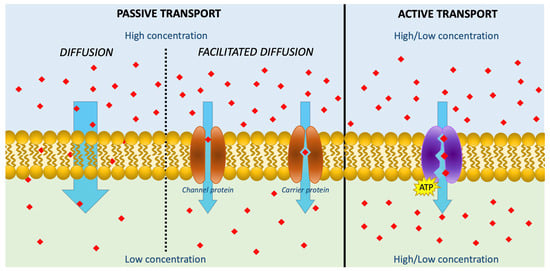
AA can be transported across membranes using three mechanisms: passive, facilitated, and active transport (Figure 1).
Figure 1. Schematic figure describing the transportation of AA across membranes using three mechanisms: passive diffusion, facilitated diffusion, and active transport. The red squares represent Vitamin C.
Passive diffusion is the most important transport route for small molecules; however, since AA is present primarily as a monoanion at a physiological pH, it is highly water-soluble, and the diffusion rate across membranes is very slow, even at high concentrations [
32]. In contrast, in the stomach and small intestine, the acid pH (1 and 5, respectively) increases the presence of unionized AA, favoring this kind of transport mechanism [
26]. Facilitated diffusion is based on carrier proteins and, as a form of passive diffusion, depends on the electrochemical gradient. For instance, thanks to its structural similarities to glucose, DHAA is absorbed through several glucose transporters, competing with glucose [
33]. The presence of this AA’s oxidized form is negligible in healthy individuals’ blood, but its intestinal concentration is probably higher because of the lack of intracellular recycling and high food concentrations. This may explain repeated results regarding the similar bioavailability of AA and DHAA [
26,
34]. However, the most important mechanism for AA membrane transport is the active one, where transporters, i.e., SVCT1 and SVCT2, play a crucial role. In the distal ileum, SVCT1 mediates the transport of AA to enterocytes, and is saturable and sodium-dependent. Thus, the absorption is not linear, and the bioavailability is maximum in low doses, while it decreases when the dose increases to above 200 mg [
10]. The uptake of AA by cells is mediated by SVCT2, a close analogue of SVCT1, sharing with it a 65% sequence homology. SVCT2 is more expressed in specialized cells in different organs and has a higher affinity but lower efficiency than SVCT1. The transport is unidirectional and based on electrochemical sodium gradients. Besides low efficiency, SVCT2 has high sensitivity, also working at low concentrations of AA. This aspect meets the physiological need for massive absorption of AA through SVCT1 from the gastrointestinal tract and the capacity of SVCT2 to uptake AA in the cells even at low plasma concentrations [
10,
26,
35,
36].
From the biological point of view, ascorbic acid is of great importance due to its antioxidant and radical scavenger properties, even if the biochemical bases of this behavior are still not understood. This antioxidant behavior is unusual, since AA donates a single reducing equivalent, forming monodehydroascorbate, which reacts preferentially with radicals instead of non-radical compounds [
37].
Ascorbic acid and its biological potential were initially discovered for the treatment of scurvy, once a very common disease, but now classified as rare; it developed during long sea voyages, when fresh fruit and vegetable stocks ran out, and without intervention typically led to death [
38,
39].
AA is essential for collagen synthesis; the enzymes procollagen-proline dioxygenase and procollagen-lysine dioxygenase, involved in the cross-linking of collagen chains, require ascorbic acid. AA does not directly participate in these reactions, but since these enzymes contain Fe
2+ as a cofactor, and it tends to oxidize to Fe
3+, inactivating the enzymes, AA is needed to reduce Fe
3+ back to Fe
2+ [
37]. AA is the cofactor of eight human enzymes; three of them participate in collagen hydroxylation, specifically the synthesis of norepinephrine, the amidation of peptide hormones, and tyrosine metabolism. A further two enzymes are involved in carnitine biosynthesis. In addition, the enzymes ascorbate oxidase and ascorbate peroxidase can reduce O
2 and H
2O
2 in H
2O, using AA as a single-equivalent donor [
32,
37,
40].
AA also plays a critical role as an antioxidant and free-radical scavenger in the non-enzymatic reduction of superoxide, hydroxyl, alkoxyl, peroxyl, tocopheroxyl and other radicals. These radicals take a single H atom from ascorbic acid, oxidizing it to monodehydroascorbate [
37]. AA, as a radical scavenger, protects cells against reactive oxygen species (ROS)’ oxidative damage, thanks to its ability to reduce ROS in stable ascorbate free radicals (AFR) serving as a one-electron donor. Then, NADH- and NADPH-dependent reductases reduce AFRs back to ascorbate inside cells. This scavenging property might explain AA’s cytoprotective function, i.e., the prevention of the oxidation-induced DNA mutation, the protection of lipids from oxidative damage, and the maintenance of protein integrity through the repair of oxidized amino acid residues [
41].
Besides its antioxidant action, AA can also exhibit pro-oxidant effects. It exerts biological protection against free transition metal ions in solutions, such as iron or copper, reducing Fe
3+ to Fe
2+, then re-oxidize producing superoxide, H
2O
2, and hydroxyl radical via Fenton chemistry. Furthermore, AA oxidizes itself in monodehydroascorbate, reducing quinones to semiquinones, which can then reoxidize in superoxide. Superoxide is then reduced by AA and monodehydroascorbate in H
2O
2 [
37,
42].
Thanks to all these properties, vitamin C is extensively used for the prevention and/or the treatment of chronic and acute pathologic conditions like diabetes, cataracts, glaucoma, macular degeneration, atherosclerosis, stroke, heart diseases, and cancer [
2].
AA is also well known as an immune system enhancer. Leukocytes accumulate AA against the gradient, resulting in an inner concentration 50 or 100 times higher than the plasma level, and their saturation could be reached with a dietary intake of ~100 mg/day, conferring a protective effect on these cells [
1]. Neutrophils internalize vitamin C via SVCT2, enhancing their chemotactic and microbial killing ability and protecting them from oxidative damage. Lymphocytes accumulate AA through SVCT; even though its action in these cells is still unclear, it surely exerts an antioxidant effect. Vitamin C promotes lymphocyte proliferation, resulting in enhanced antibody production and reduced susceptibility to cell death stimuli, i.e., exposure to toxins and chemicals. In addition, AA induces T and natural killer cell differentiation and maturation [
43].
In recent years, with the outbreak of the COVID-19 pandemic, vitamin C has been proposed as a supplement in treatment and prevention protocols for its antioxidant and immune enhancer properties, but also for its safety profile and low cost [
44,
45].
Intravenous injection of high doses of vitamin C has a cytotoxic effect on cancer cells, and many ongoing clinical trials are exploring its safety and efficacy in treating various types of cancer as a monotherapy or combination therapy. Cancer cells are generally more sensitive to oxidative stresses than normal cells for their upregulated metabolism and defective mitochondria [
46]. There are many hypotheses posited to explain the anticancer effect of vitamin C. One of them is that pharmacological concentrations of AA cause a pro-oxidant effect, stimulating the formation of hydrogen peroxide and ROS, which have a direct cytotoxic activity on cancer cells. This mechanism is even enhanced by the presence of redox-active transition metals, such as iron, inside the cancer cells. In addition, after administration, AA is oxidized in DHAA and, thanks to its structural similarities with glucose, it is internalized by the upregulated glucose transporters of cancerous cells. Once inside the cell, the DHAA is reduced back to AA at the expense of intracellular antioxidants, increasing the endogenous levels of ROS [
46,
47]. Another hypothesis is based on the epigenetic changes induced by AA due to its effect on 2-oxoglutarate-dependent dioxygenases, like histone and DNA demethylases [
48,
49]. In addition, DNA demethylation, mediated by the ten-eleven translocation enzymes activation, allows the re-expression of tumor-suppressor genes in cancer cells such as p53, promotion of stem cell differentiation, inhibition of leukemogenesis, and enhancement of DNA methyltransferase inhibitor-induced immune signals via increased expression of endogenous retrovirus transcripts. AA also plays a role in cancer treatment by enhancing chemo and radiosensitivity and decreasing the side effects by acting as a prodrug for hydrogen peroxide [
50,
51]. Furthermore, it can enhance the efficacy of immunotherapy, modulating cytokine generation, improving T cell responses by reverting their exhaustion-associated DNA methylation program and helping to overcome resistance to the immune checkpoint blockade [
52,
53].
AA has also been proposed for the treatment of patients with septic shock, and its parenteral administration with this aim has undergone many clinical trials. In addition to its antioxidant and scavenging properties, vitamin C acts as a cofactor for dopamine b-hydroxylase and tyrosine hydroxylase, increasing the endogenous production of norepinephrine, dopamine, and vasopressin, usually administered to manage hypotension refractory to catecholamines, which is a hallmark of sepsis. In addition, it may improve and preserve microvascular function, contribute to endothelial cell proliferation and apoptosis, smooth-muscle-mediated vasodilation, and endothelial barrier permeability; attenuate neutrophil necrosis; and demonstrate bacteriostatic activity [
54,
55].
3. AA in Cosmetics
Besides the oral route, ascorbic acid can also be administered topically for skin health applications, since it is involved in skin synthesis, depigmentation, and antioxidant activity.
Thanks to its high aqueous solubility, AA is available in the aqueous compartments of the cell; thus, skin application is one of its most established functions. It acts as a potent antioxidant by neutralizing and removing oxidants produced by the skin’s interactions with environmental pollutants or after ultraviolet light exposure, especially in the epidermis, wherein vitamin C is more concentrated [
56]. Repeated exposure to UV light reduces the availability of AA in the skin. Although it has no UV absorption spectra in the UVA (320 to 400 nm) or UVB (290 to 320 nm) range, topical administration of AA can exert a photoprotective effect against UVR due to its antioxidant and anti-inflammatory properties [
30].
After wounding, the vitamin C level in the injured site decreases dramatically due to depletion generated by free oxidant radicals and its increased consumption in biological repair processes. Apart from the antioxidant action in contrasting oxidative stress, AA assists fibroblasts in producing and cross-linking a stable form of collagen, providing a strong framework for repair; it also suppresses pro-inflammatory processes and promotes anti-inflammatory and pro-resolution effects in macrophages [
57,
58].
Furthermore, AA is also used in cosmetics as an antipigmentary agent, through interacting with the copper ions of tyrosinase enzymes, which converts tyrosine into melanin, thereby inhibiting its action in some conditions such as skin hyperpigmentation, melasma, or age spots [
30,
59].
However, AA is chemically active and unstable in aqueous media, as it is easily oxidizable or decomposable, very prone to photooxidation, and its skin absorption is limited. For these reasons, AA’s topical efficacy has been improved using different approaches. The first approach is to use some hydrophobic AA precursors, which can increase AA’s concentration in the cytoplasm, easily crossing the cell membrane [
60], or in AA glycosides, which act as a reservoir of AA thanks to its continuous release due to enzymatic hydrolysis [
61]. The second approach is the application of technologies to cosmetics, such as the encapsulation of AA inside reverse micelles, nanoparticles, or liposomal formulations, which can maintain its stability and improve its delivery to the target site [
62,
63].
4. AA Industrial Applications
AA’s strong antioxidant activity has encouraged the food industry to develop some additives based on this compound. Nowadays, there are several commercial food additives derived from AA: E300, ascorbic acid; E301, sodium ascorbate; E302, calcium ascorbate; E303, potassium ascorbate; and E304, fatty acid esters of ascorbic acid (ascorbyl palmitate and ascorbyl stearate). They are used in the production and transformation phases of several foods such as beer, gelatines, jam, sweets, bread and baked products, fruit juices, wine, fishing products, and meats. AA in foods usually prevents nitrosation and oxidation, as well as the discoloration of products during storage, which could be unpleasant for the consumers but is not associated with organoleptic alterations [
64,
65].
In addition, AA is also used for animal feed, with the aim of body weight gain, feed efficiency, nutrient digestibility, immune response, and antioxidant status [
66,
67,
68].
AA is widely used in the chemical industry as additive, as it is a weak reducing agent thus can suppress the oxidation of desired compounds [
69,
70,
71,
72]; it can also be used as a leaching agent for valuable metals [
73,
74].