1. Biochar Properties
The most relevant properties of biochars in relation to their adsorption ability are the specific surface area (i), the pore size distribution (ii), and surface functional groups (iii) such as hydroxyl, carboxyl, carbonyl, etc. [
68]. The mineral content and composition (iv) of biochars are also important in the adsorption of bulky antibiotics such as tetracyclines through surface complexation [
53,
69].
The biochar’s properties contribute to the available active sites for adsorption, improving the adsorption capacity [
70]. The pore structure is formed due to the release of volatile compounds and water loss in the dehydration process during pyrolysis. Thus, the feedstock and the pyrolysis conditions, especially the temperature, significantly affect the biochar’s pore structure and, consequently, the adsorption capacity of the biochar [
49,
71,
72].
A high pyrolysis temperature has been linked to a larger surface area, higher microporosity, and graphitic structures, due to the increase in volatilization at higher temperatures [
73,
74,
75,
76,
77]. On the other hand, at low pyrolysis temperatures, the functional groups are retained and they contribute to adsorption [
69,
78]. Therefore, generally, moderate temperatures (400–700 °C) are more suitable for the development of favorable pore structures [
74]. The aromatic carbon groups (C=C), carbonyl groups (C=O), and aliphatic groups (CH
2 + CH
3) were determined for four biochars produced by carbonizing corn crop residue (
Zea mays L.) and wood shavings of oak (
Quercus ssp.) at 350 °C and 600 °C using slow pyrolysis. The results showed that the aromatic carbon content increased with temperature for both biochars, while the carbonyl and aliphatic groups decreased [
79,
80], which is in agreement with the results of Fu et al. [
81]. Thus, a high pyrolysis temperature is almost always advantageous for the adsorption process, although the biochar yield decreases with increasing pyrolysis temperature and the economic viability of the process is reduced.
Several pore measurements have been reported for biochars. The most common measure is the total pore volume, which includes all pores. According to the International Union of Pure and Applied Chemistry (IUPAC), pores can be divided into three main groups: micropores (<2 nm), mesopores (2–50 nm), and macropores (>50 nm) [
71]. However, some authors also use the term “nanopores” to indicate micropores, probably because they are in the nanometer range. The role of each type of pore in adsorption is different. Macropores are primarily linked to the diffusion of substances, mesopores serve as channels for mass transfer, and micropores provide space for trapping [
71,
82]. The high temperatures in pyrolysis have been stated to be responsible for the presence of pores with sizes around 1.2 and 1.0 nm—the so-called micropores—leading to an increased surface area [
83]. Nevertheless, the surface area only increases with the pyrolysis temperature up to a maximum, after which the surface area decreases [
68]. For instance, some authors state that there are two competing phenomena: the first increases the volatile release and, consequently, the surface area; and the other is thermal deactivation that leads to char melting, pore fusion, and structure ordering, which decrease the surface area and pore volume [
84,
85,
86].
The heating rate is also important in the formation of the pore structure. For example, tests conducted at two different heating rates (10–30 °C/min and 50 °C/min) showed that at the lower heating rate the volatiles formed were released from the surface, leading to an open fiber structure with the formation of cavities and, therefore, increasing the surface area [
87]. On the other hand, a higher heating rate led to a decrease in surface area and pore volume, which was believed to be due to some of the pore walls becoming too thin and breaking [
87]. The same effect was also observed with pyrolysis residence time. Thus, the severity of the pyrolysis conditions (i.e., maximum pyrolysis temperature, heating rate, and solid residence time) increases the surface area to some extent, but it decreases after a certain limit (which is dependent on biomass, chemical and anatomical composition, and the heat and mass transfer rate). This phenomenon has two practical implications: (i) it is not always necessary to apply the most severe conditions, and (ii) energy savings can be achieved by applying the optimal pyrolysis conditions.
The determination of the surface area available for adsorption faces some problems. For instance, the prevailing method to determine the surface area, N
2 adsorption at 77 K, has a kinetic diffusion limitation for N
2 in small micropores [
88]. The kinetic limitation arises from the inflexibility of the matrix, leading to an artificially lower surface area for some chars. This phenomenon has been reported by several authors, for instance for oak, pine, and grass chars, where the N
2 surface area was 225, 285, and 77 m
2/g, respectively, while the CO
2 area for the same materials was 528, 843, and 427 m
2/g, respectively [
89]. Similar results were presented for sewage sludge and wood chip char [
90]. The higher surface area estimation by CO
2 has been reported to be due to the higher kinetic energy associated with the smaller kinetic diameter of CO
2 (3.3 Å vs. 3.64 Å for CO
2 and N
2, respectively), which allows CO
2 to diffuse more easily into the small pores [
89,
91,
92].
Argon has also been used to measure char’s surface area at 77 K and 87 K. The results showed that at 87 K the surface area was slightly greater than at 77 K, which was attributed to the increased mobility of Ar molecules at higher temperatures. On the other hand, the low values of surface area measured by Ar were believed to be due to the lower amount of mesopores [
93].
The size of the pores also affects the sorption, because the filling of micropores involves a higher number of contact points than the filling of mesopores, and pore filling has been characterized as being influenced by size exclusion effects [
88]. A comparison of the adsorption-relevant properties of different biochars is presented in
Table 2.
Table 2. Pyrolysis temperature, char yield, surface properties, and ash content of different biochars.
The results of Table 2 show that biochar properties are highly variable between different precursors and applied pyrolysis temperatures. However, data analyses allow for certain conclusions:
-
The specific surface area of biochars is usually between 0 and 100 m2/g;
-
Wood biochars have the greatest specific surface area (up to 738 m2/g);
-
The pore volume of different biochars is between 0 and 0.2 cm3/g;
-
The ash content of biochars is highly variable; it is highest in sewage sludge, algae, and manure biochars, and lowest in wood biochars.
2. Modification of Biochars
A number of methods have been developed to tailor and maximize the adsorption capacity of biochars used in water treatment and soil remediation, as well as in energy storage [
139]. The modified or engineered biochar is the derivative of pristine biochar that has undergone physical, chemical, or biological treatments to improve its properties, such as its specific surface area, porosity, cation-exchange capacity, surface functional groups, pH, etc. [
140,
141,
142]. The engineered biochars contain a large number of carbons, including activated carbons. Interestingly, most biochar engineering methods are less expensive and easier processes than the typical carbon activation processes [
68].
Currently, different physical or chemical modifications (
Table 3) are applied to biochars to improve their adsorption capacity [
12]. These modifications are discussed below.
Table 3. Critical comparison of the advantages and disadvantages of the main biochar modification methods.
Acid or alkali activation is the most widely used and effective way to enhance biochars’ surface area and porosity. Both acid and alkali treatments increase the porosity of biochars by altering the biochar’s structure and surface functional groups via depolymerization, dehydration, and dehydrogenation reactions (i), creating micro- and mesopores inside the biochar’s structure (ii), and removing the inorganic compounds (iii) [
49,
143,
144,
145].
Acid–base combined treatments can be considered for low-porosity biochars bearing limited surface functional groups, such as municipal sewage sludge biochars [
49,
146,
147]. These treatments seem to be superior to the single acid or alkali treatments [
49]. However, the available data are still scarce. More experimental results with a broader range of biochars are required to better understand the effects of the combined acid–base treatments.
Physical treatment methods, such as coating with carbonaceous materials, ball milling, and template formation, can also result in surface enhancement. Ball milling seems to be a feasible method to produce biochar nanoparticles [
148]. Future research should focus on developing technologies to simultaneously achieve enhanced functionality and porous structure of biochars.
Cationic or anionic surfactants such as cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) are used to alter the adsorbent’s surface and, in particular, to change the surface charge [
149]. Certain organic compounds, such as humic acid (HA) [
150], methanol [
151], and chitosan [
152,
153,
154], have been used in the modification of biochars because they introduce supplementary functional groups (e.g., carbonyl (-C=O-), amino (−NH
2) and hydroxyl (-OH)) to the surface of biochar [
49]. However, organic compound modification has cost disadvantages, which limit its development [
49]. Metal or metal oxide modification provides a higher number of adsorption sites and creates a larger surface area in biochars [
49,
146,
155,
156,
157]. The metal modification is particularly effective in the recycling of biochars after adsorption. However, metal modifications generate contamination of water bodies through metal ion shedding [
49].
Doping with carbonaceous materials is the introduction of carbonaceous materials (e.g., graphene and carbon nanotubes) into the surface structure of biochars to improve their adsorption efficiency [
49,
158]. The increased number of adsorption sites and the increased specific surface area of the biochar improve its adsorption capacity [
49,
159,
160]. However, graphene, graphene oxide, and carbon nanotubes are highly expensive materials and cannot be considered practical for large-scale adsorption applications [
49].
Non-metallic or heteroatom doping of biochars using nitrogen [
161,
162,
163], oxygen [
162], sulfur [
163], or phosphorus [
162] is an efficient modification method to offer increase the stability and adsorption efficiency of adsorbents [
49]. The heteroatom doping of biochar provides additional surface functional groups and active sites for adsorption. However, the available research is currently scarce [
49].
Other physical modifications, such as steam activation [
164] and ball milling [
158,
165], generate a higher specific surface area, a higher number of functional groups, and pores in biochars. Physical modifications are environmentally friendly, as they do not use any chemicals during biochar modification [
49]. However, they are comparatively less effective than chemical modifications [
49].
Molecular imprinting improves the specific adsorption of biochars by creating selective active sites [
49]. Molecularly imprinted biochars can be used to remove low-concentration and highly toxic pollutants [
49,
166]. Molecularly imprinted biochars have already been used to detect and quantify antibiotic residues at trace levels in food and environmental samples [
49,
167,
168]. Molecularly imprinted biochars are reusable, which is their major advantage compared to other biochars [
48]. Similar to other modified biochars, molecularly imprinted biochars usually exhibit better adsorption properties for antibiotics than pristine biochars [
49].
3. Biochar-Based Antibiotic Adsorption Studies
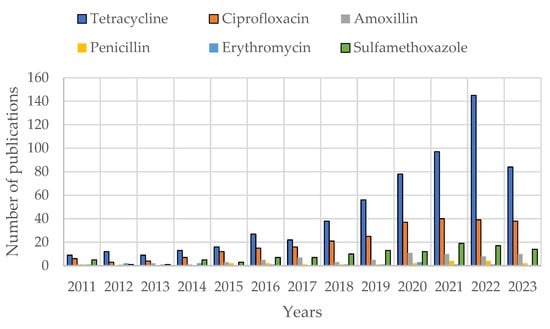
In the bibliometric survey section, it was shown that antibiotic adsorption studies are increasing in number, while in the antibiotics and bacterial cytology section, antibiotics were grouped into different classes based on their chemical structure. It is important to observe the adsorption studies of each major antibiotic group. Figure 3 provides an overview of adsorption studies with different antibiotics. Adsorption studies were predominantly performed on tetracycline, fluoroquinolone, and sulfonamide antibiotics. This is consistent with the co-occurrence map (Figure 2) and suggests that these antibiotics are selective to carbonaceous adsorbents.
Figure 3. Number of publications with “adsorption” and “different antibiotics” titles on the Web of Science (WOS).
Previous studies on biochar-based removal of antibiotics were mainly performed on modified biochars. The usage of pristine biochars for the removal of antibiotics is currently limited to biochars prepared by pyrolysis, which are termed pristine biochars (PBs) [
68]. However, in order to modify the adsorption performance of biochars, the first step is to understand the adsorption performance of pristine biochars by studying the biochars’ properties and adsorption mechanisms.
The adsorption mechanisms of different carbonaceous materials are not identical, although certain mechanisms, such as π–π electron donor–acceptor (EDA) interactions and hydrogen bonding, are considered both for high-surface-area carbon nanotubes and for biochars, indicating the role of intermolecular interactions in the adsorption [
169]. According to Du et al. (2023), at least seven different mechanisms, including hydrogen bonding, π–π interactions, surface complexation, electrostatic interactions, pore filling, ion exchange, and hydrophobic interactions, can contribute to the adsorption of antibiotics onto biochars [
49]. This excellent review also showed that antibiotic adsorption studies with biochars were mostly performed with modified biochars (approximately 65% of the studies) [
49].
Table 4 provides a comparison of proposed antibiotic adsorption mechanisms and maximum adsorption capacities for pristine biochars and modified biochars.
Table 4. Mechanisms of antibiotic adsorption onto biochars; modified from [
12].
The results of Table 4 suggest that the modification of biochars significantly affects their antibiotic adsorption capacity. The antibiotic adsorption mechanisms were principally studied on modified biochars, and π–π interactions were the most commonly proposed mechanisms for both types of biochars.
4. Thermodynamic and Kinetic Considerations
Thermodynamic and kinetic (rate and mechanism) studies are the two essential tools in adsorption studies because they answer fundamental questions such as whether an adsorption process works, how it works, how to optimize it, and how to design better adsorbents.
Thermodynamics determines the feasibility of an adsorption process under various temperature and pressure conditions. The thermodynamic analysis of adsorption involves the calculation of thermodynamic parameters such as Gibbs free energy, enthalpy, and entropy (ΔG, ΔH, and ΔS, respectively), which can be used to assess the thermodynamic feasibility of the adsorption process. For instance,
- -
-
If ΔG < 0, the process is thermodynamically favorable, and adsorption will occur spontaneously;
- -
-
If ΔG > 0, the process is thermodynamically unfavorable, and adsorption will not occur spontaneously:
- -
-
If ΔG = 0, the process is at thermodynamic equilibrium.
Enthalpy change (ΔH) is another thermodynamic parameter that is used to assess the feasibility of adsorption. If the adsorption is exothermic (ΔH < 0), it releases heat and is more favorable at lower temperatures. If the adsorption is endothermic (ΔH > 0), it absorbs heat and is more favorable at higher temperatures. Entropy change (ΔS) is the final factor to assess the feasibility of adsorption. An increase in entropy (ΔS > 0) favors the adsorption process.
These factors are related according to the following equation:
In order for ΔG to be negative, either the enthalpy change (ΔH) should be negative (exothermic process) and greater than the TΔS product (typically positive), or, in the case of endothermic reactions, the entropy change (ΔS) should be large enough to offset the positive enthalpy change (ΔH) and temperatures (T) should be high.
Exothermic adsorption (ΔH < 0) involves relatively strong adsorbate–surface interactions, such as chemical sorption or strong van der Waals forces, while endothermic adsorption involves weak adsorbate–surface interactions such as physical sorption (weak intermolecular interactions). The entropy change (ΔS) may be positive or negative in chemical sorption, but it is usually negative in physical sorption.
Pressure can also affect the adsorption process, but its impact in liquid adsorption is less pronounced than temperature. High pressures increase the entropy and favor the adsorption process, but they may also lead to degradation of the adsorbent.
Thus, in order to optimize the adsorption of antibiotics onto biochars, it is necessary to calculate the thermodynamic properties. If the adsorption is exothermic, it should be performed at low temperatures, and if the adsorption is endothermic it should be performed at high temperatures. If the adsorption occurs due to surface chemical reactions, the adsorbent’s surface should be modified with metal oxides or heteroatoms to increase the number of available complexation sites and drive the adsorption process in a thermodynamically favorable manner. If the adsorption occurs through physical sorption, oxygenated surface functional groups should be introduced to the biochars to increase the intermolecular interactions, such as hydrogen bonds. It should be noted that the adsorption of antibiotics is a complex reaction and involves both surface chemical reactions and physical sorption [
172]. Therefore, different experimental conditions should be tested to optimize the adsorption process.
A particular case of thermodynamic studies is the study of adsorption isotherms that describe the equilibrium relationship between the concentration of adsorbate molecules and the amount of adsorbate adsorbed onto the surface of the adsorbent. Thus, they provide information about the adsorption capacity and the adsorbent–adsorbate surface interactions. The Langmuir isotherm and Freundlich isotherm are the most frequently used adsorption isotherms. According to the Langmuir isotherm, the adsorbent’s surface is homogeneous, and adsorption occurs as a monolayer until all available sites are occupied by adsorbate molecules and there are no interactions among the adsorbed molecules. The Freundlich isotherm assumes a heterogeneous adsorbent surface, multilayer adsorption, and interactions among the adsorbed molecules.
Kinetic models of adsorption describe how adsorbate molecules are adsorbed onto the surface of an adsorbent material as a function of time. The kinetic models explain the reaction rate and the mechanisms, and they provide insights into the dynamic aspects of adsorption. The most frequently used kinetic models are the pseudo-first-order model, pseudo-second-order model, Elovich model, and intraparticle diffusion model. The pseudo-first-order and pseudo-second-order models consider the surface reaction as the rate-liming step, while the intraparticle diffusion model considers the intraparticle diffusion as the rate limiting step. According to the pseudo-first-order kinetic model, the adsorption rate (dq/dt) is proportional to the difference between the equilibrium concentration (qe) and the concentration at a given time (q), while according to the pseudo-second-order kinetic model the adsorption rate is proportional to the square difference between the equilibrium concentration and the concentration at a given time. The Elovich model assumes that rate of the adsorption is not constant over time and that there are interactions between the adsorbate molecules. The intraparticle diffusion model describes the rate of intraparticle diffusion.
The adsorption of tetracycline antibiotics onto zinc chloride activated biochar was described by the pseudo-second-order kinetic model and the Langmuir isotherm, with a maximum (monolayer) adsorption capacity of 200 mg/g tetracycline. Hydrogen bonding and electrostatic interactions were the main proposed mechanisms [
176]. The adsorption of quinolone antibiotics onto magnetic biochar also resulted in a similar trend. The adsorption was described by the pseudo-second-order kinetic model and the Langmuir isotherm, with a maximum adsorption capacity of 68.9 mg/g [
177]. Similarly, the adsorption of tetracycline, quinolone, and sulfonamide antibiotics onto H
3PO
4 activated biochar was described well by the Elovich and pseudo-second-order kinetic models, as well as by the Langmuir isotherm [
172]. Interestingly the results of this latter study indicated that the adsorption of antibiotics is an endothermic and spontaneous process with negative Gibbs free energy and a positive entropy change. Both chemical and physical adsorption occurred simultaneously [
172]. The endothermic character of antibiotic adsorption on activated carbon was also reported for the adsorption of heavy metals [
178]. On the other hand, the adsorption of sulfonamide antibiotics onto H
3PO
4 activated biochar resulted in a spontaneous and exothermic process that was favorable at low temperatures [
179]. The adsorption was described by the Langmuir isotherm and the pseudo-second-order kinetic model, similar to previous studies [
179]. The study of Srivastava et al. (2002) also showed a similar trend. The adsorption of quinolone and tetracycline antibiotics onto modified biochar was exothermic and was described by the Langmuir isotherm and the pseudo-second-order kinetic model [
180].
The above examples were the modified biochars, which are the biochars most frequently used as adsorbents. The kinetic models and isotherms for the adsorption of antibiotics onto pristine biochars seem to follow the same trend (i.e., Langmuir isotherm and pseudo-second-order kinetics). However, there are still few studies on pristine biochars providing insights into their adsorption mechanisms. For instance, the adsorption of tetracycline antibiotics onto wheat-stalk biochars was described well with the Langmuir isotherm as well as the pseudo-second-order and intraparticle diffusion kinetic models [
148]. A similar kinetic model was reported for the adsorption of sulfonamide antibiotics onto a biochar based on spent coffee grounds. The adsorption kinetics of sulfadiazine (SDZ) and sulfamethoxazole (SMX), two common sulfonamide antibiotics, was better described by a pseudo-second-order model, implying that the adsorption of antibiotics onto biochars is controlled by the chemisorption mechanism [
171].
The overall results indicate that the adsorption of antibiotics onto pristine or modified biochars predominantly occurs as a monolayer through surface reactions and intraparticle diffusion mechanisms. Thermodynamic studies of the adsorption of different antibiotics have shown that the adsorption of antibiotics on biochars can be exothermic or endothermic and should be determined for each adsorption case to improve the adsorption efficiency.
This entry is adapted from the peer-reviewed paper 10.3390/app132111963