It is estimated that 90% of brain injuries fall into the category of mild traumatic brain injury (TBI). Mild TBI and concussion can have heterogeneous symptoms and serious consequences that develop over time with unpredictable levels of recovery. The diagnosis may not be made for multiple reasons, including due to patient hesitancy to undergo neuroimaging and inability of imaging to detect minimal damage. Biomarkers of nerve damage measured in blood plasma are increasingly promising as the sensitivity and accuracy of quantification improves. These biomarkers could fill the gap, making detection, diagnosis and prognosis easier and more precise, but the time needed to send blood to a laboratory for analysis made this impractical until point-of-care measurement became available. A handheld blood test is now on the market for diagnosis of concussion based on the specific blood biomarkers glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl terminal hydrolase L1 (UCH-L1). Other blood biomarker assays are in development.
- concussion
- mild traumatic brain injury
- biomarker
- diagnosis
1. Introduction
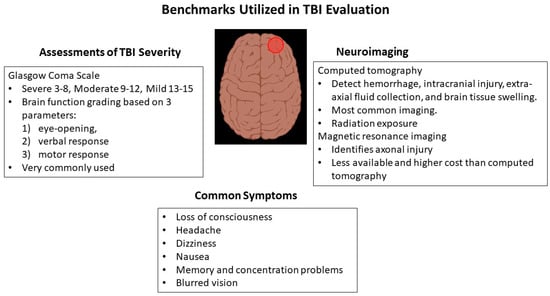
2. Current Modes of Mild TBI Evaluation
2.1. Glasgow Coma Scale
2.2. Imaging by Computed Tomography
2.3. Magnetic Resonance Imaging
2.4. Evaluating Concussion: Emphasis on Sports-Related Concussion
3. Point-of-Care Cognitive Tests for Mild TBI
4. Blood Biomarkers for Mild TBI
5. Potential Expanded Use of Point-of-Care Biomarker TBI Testing
Persons who have suffered a TBI and reach an emergency department or other facility equipped with the analyzer within the delimited time period are considered candidates for Abbott’s new Alinity i TBI test. The biomarker levels in these patients could serve as a source of valuable data, whether or not the CT scan is obtained in tandem. The presence or levels of GFAP and UCH-L1 cannot reliably discriminate between patients who will improve rapidly versus those with prolonged recovery time who may continue to experience troubling neurologic symptoms [58]. Currently, there is no test with that capability [59][60]. This poses a challenge, especially as a majority of TBI patients have no follow-up scheduled. A positive blood test might suggest that follow-up care is indicated, but scholars propose that TBI biomarker data obtained where healthcare is provided such as the Alinity i TBI test can give the medical community so much more. Analysis and integration of data aggregated in emergency departments performing TBI biomarker testing could produce a massive database that would allow the development of a predictive model so that those at the highest risk for poor recovery could be targeted for early intervention. Sports provide a unique advantage in data collection with an opportunity to obtain preseason baseline tests so that within-player change in plasma biomarkers with TBI could be accurately captured [61].
In addition, the lack of effective treatment for mild TBI beyond rehabilitation and symptomatic relief makes the idea of applying the Alinity i TBI test data to acquire a more complete understanding of damage development very appealing [62][63]. This type of understanding is necessary if a breakthrough is to come, whether through the repurposing of current drugs or the application of new gene-expression-altering technologies to interrupt a program of neuronal destruction set in motion by the head injury [64] (Figure 2).
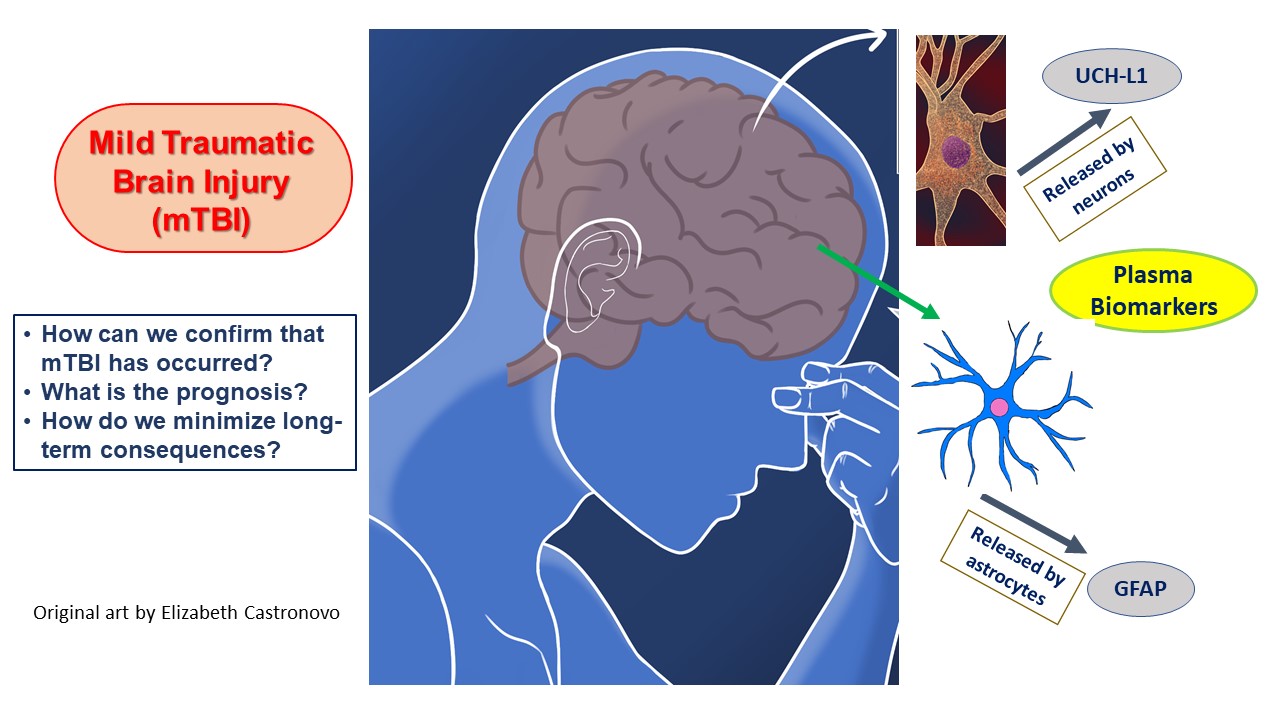
Figure 2. The blood biomarkers utilized in the Alinity i TBI test and delineates the major issues involved in mild TBI diagnosis and management.
Yet another consideration in biomarker use in TBI is the lack of any validated assessment for those under age 18. The absence of representation of the pediatric concussion patient is a major gap since the developing brain is particularly susceptible to injury and prolonged sequelae [65][66]. In older persons, baseline levels of biomarkers are higher, and discriminative capability may be less or of shorter duration after injury [60][67][68]. In persons with Alzheimer’s disease, GFAP levels may be elevated [69]. The need to devise age-appropriate TBI biomarker tests is clear.
The value of the Alinity I test in TBI patients who present after the recommended testing window is somewhat in question. Although biomarker levels lose their ability to distinguish who should receive imaging, that does not preclude using the data to develop a model that will give us prognostic information concerning the chance that long-term neurologic consequences will occur [47][70][71].
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13213330
References
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238.
- Nguyen, J.V.K.; McKay, A.; Ponsford, J.; Davies, K.; Makdissi, M.; Drummond, S.P.A.; Reyes, J.; Willmott, C. Interdisciplinary Rehabilitation for Concussion Recovery (i-RECOveR): Protocol of an investigator-blinded, randomised, case series with multiple baseline design to evaluate the feasibility and preliminary efficacy of a 12-week treatment for persistent post-concussion symptoms. Pilot Feasibility Stud. 2022, 8, 198.
- Cicuendez, M.; Castaño-León, A.; Ramos, A.; Hilario, A.; Gómez, P.A.; Lagares, A. Prognostic value of corpus callosum injuries in severe head trauma. Acta Neurochir. 2017, 159, 25–32.
- Bruggeman, G.F.; Haitsma, I.K.; Dirven, C.M.F.; Volovici, V. Traumatic axonal injury (TAI): Definitions, pathophysiology and imaging-a narrative review. Acta Neurochir. 2021, 163, 31–44.
- Hill, C.S.; Coleman, M.P.; Menon, D.K. Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends Neurosci. 2016, 39, 311–324.
- Risling, M.; Smith, D.; Stein, T.D.; Thelin, E.P.; Zanier, E.R.; Ankarcrona, M.; Nilsson, P. Modelling human pathology of traumatic brain injury in animal models. J. Intern. Med. 2019, 285, 594–607.
- Bigler, E.D. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2004, 10, 794–806.
- Mena, J.H.; Sanchez, A.I.; Rubiano, A.M.; Peitzman, A.B.; Sperry, J.L.; Gutierrez, M.I.; Puyana, J.C. Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: Comparing classic and modified Glasgow Coma Scale score model scores of 13. J. Trauma 2011, 71, 1185–1193.
- Åkerlund, C.A.I.; Holst, A.; Stocchetti, N.; Steyerberg, E.W.; Menon, D.K.; Ercole, A.; Nelson, D.W.; CENTER-TBI Participants and Investigators. Clustering identifies endotypes of traumatic brain injury in an intensive care cohort: A CENTER-TBI study. Crit. Care 2022, 26, 228.
- Chong, C.D.; Schwedt, T.J. Research Imaging of Brain Structure and Function After Concussion. Headache 2018, 58, 827–835.
- Rauchman, S.H.; Albert, J.; Pinkhasov, A.; Reiss, A.B. Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System. Neurol. Int. 2022, 14, 453–470.
- Ganti, L.; Daneshvar, Y.; Bodhit, A.; Ayala, S.; Patel, P.S.; Lottenberg, L.L.; York, D.; Counsell, C.; Peters, K.R. TBI ADAPTER: Traumatic brain injury assessment diagnosis advocacy prevention and treatment from the emergency room--a prospective observational study. Mil. Med. 2015, 180, 380–386.
- Melnick, E.R.; Szlezak, C.M.; Bentley, S.K.; Dziura, J.D.; Kotlyar, S.; Post, L.A. CT overuse for mild traumatic brain injury. Jt. Comm. J. Qual. Patient Saf. 2012, 38, 483–489.
- Kim, J.J.; Gean, A.D. Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics 2011, 8, 39–53.
- Svensson, S.; Vedin, T.; Clausen, L.; Larsson, P.A.; Edelhamre, M. Application of NICE or SNC guidelines may reduce the need for computerized tomographies in patients with mild traumatic brain injury: A retrospective chart review and theoretical application of five guidelines. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 99.
- Vidhya, V.; Gudigar, A.; Raghavendra, U.; Hegde, A.; Menon, G.R.; Molinari, F.; Ciaccio, E.J.; Acharya, U.R. Automated Detection and Screening of Traumatic Brain Injury (TBI) Using Computed Tomography Images: A Comprehensive Review and Future Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 6499.
- Nayebaghayee, H.; Afsharian, T. Correlation between Glasgow Coma Scale and brain computed tomography-scan findings in head trauma patients. Asian J. Neurosurg. 2016, 11, 46–49.
- Maas, A.I.; Hukkelhoven, C.W.; Marshall, L.F.; Steyerberg, E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005, 57, 1173–1182.
- Deepika, A.; Prabhuraj, A.R.; Saikia, A.; Shukla, D. Comparison of predictability of Marshall and Rotterdam CT scan scoring system in determining early mortality after traumatic brain injury. Acta Neurochir. 2015, 157, 2033–2038.
- Nelson, D.W.; Nyström, H.; MacCallum, R.M.; Thornquist, B.; Lilja, A.; Bellander, B.M.; Rudehill, A.; Wanecek, M.; Weitzberg, E. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J. Neurotrauma 2010, 27, 51–64.
- Raj, R.; Siironen, J.; Skrifvars, M.B.; Hernesniemi, J.; Kivisaari, R. Predicting outcome in traumatic brain injury: Development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery 2014, 75, 632–646.
- Pozzato, I.; Meares, S.; Kifley, A.; Craig, A.; Gillett, M.; Vu, K.V.; Liang, A.; Cameron, I.; Gopinath, B. Challenges in the acute identification of mild traumatic brain injuries: Results from an emergency department surveillance study. BMJ Open 2020, 10, e034494.
- Valérie Boucher; Jérôme Frenette; Xavier Neveu; Pier-Alexandre Tardif; Éric Mercier; Jean-Marc Chauny; Simon Berthelot; Patrick Archambault; Jacques Lee; Jeffrey J. Perry; et al. Lack of association between four biomarkers and persistent post-concussion symptoms after a mild traumatic brain injury. J. Clin. Neurosci. 2023, 118, 34-43.
- Freire-Aragón, M.D.; Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J. Update in mild traumatic brain injury. Actualización en el traumatismo craneoencefálico leve. Med. Clin. 2017, 149, 122–127.
- Feinberg, C.; Mayes, K.D.; Portman, E.; Carr, C.; Mannix, R. Non-invasive fluid biomarkers in the diagnosis of mild traumatic brain injury (mTBI): A systematic review. J. Neurol. Neurosurg. Psychiatry. 2023. Advance online publication.
- Mikolic, A.; Steyerberg, E.W.; Polinder, S.; Wilson, L.; Zeldovich, M.; von Steinbuechel, N.; Newcombe, V.; Menon, D.; van der Naalt, J.; Lingsma, H.F.; et al. Prognostic models for global functional outcome and post-concussion symptoms following mild traumatic brain injury: A CENTER TBI study. J. Neurotrauma 2023, 40, 1651–1670.
- Yousaf, T.; Dervenoulas, G.; Politis, M. Advances in MRI Methodology. Int. Rev. Neurobiol. 2018, 141, 31–76.
- Wang, M.L.; Li, W.B. Cognitive impairment after traumatic brain injury: The role of MRI and possible pathological basis. J. Neurol. Sci. 2016, 370, 244–250.
- Vaisvilaite, L.; Hushagen, V.; Grønli, J.; Specht, K. Time-of-day effects in resting-state functional magnetic resonance imaging: Changes in effective connectivity and blood oxygenation level dependent signal. Brain Connect. 2022, 12, 515–523.
- Massaad, E.; Shin, J.H.; Gibbs, W.N. The Prognostic Role of Magnetic Resonance Imaging Biomarkers in Mild Traumatic Injury. JAMA Netw. Open 2021, 4, e211824.
- Gonzalez, A.C.; Kim, M.; Keser, Z.; Ibrahim, L.; Singh, S.K.; Ahmad, M.J.; Hasan, O.; Kamali, A.; Hasan, K.M.; Schulz, P.E. Diffusion Tensor Imaging Correlates of Concussion Related Cognitive Impairment. Front. Neurol. 2021, 12, 639179.
- Grassi, D.C.; Conceição, D.M.D.; Leite, C.D.C.; Andrade, C.S. Current contribution of diffusion tensor imaging in the evaluation of diffuse axonal injury. Arq. Neuropsiquiatr. 2018, 76, 189–199.
- De Luigi, A.J.; Bell, K.R.; Bramhall, J.P.; Choe, M.; Dec, K.; Finnoff, J.T.; Halstead, M.; Herring, S.A.; Matuszak, J.; Raksin, P.B.; et al. Consensus Statement: An Evidence-Based Review of Exercise, Rehabilitation, Rest, and Return to Activity Protocols for the Treatment of Concussion and Mild Traumatic Brain Injury. PMR 2023. Advance online publication.
- Li, L.; Liu, J. The effect of pediatric traumatic brain injury on behavioral outcomes: A systematic review. Dev. Med. Child Neurol. 2013, 55, 37–45.
- McCrory, P.; Feddermann-Demont, N.; Dvořák, J.; Cassidy, J.D.; McIntosh, A.; Vos, P.E.; Echemendia, R.J.; Meeuwisse, W.; Tarnutzer, A.A. What is the definition of sports-related concussion: A systematic review. Br. J. Sports Med. 2017, 51, 877–887.
- Stiell, I.G.; Wells, G.A.; Vandemheen, K.; Clement, C.; Lesiuk, H.; Laupacis, A.; McKnight, R.D.; Verbeek, R.; Brison, R.; Cass, D.; et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001, 357, 1391–1396.
- Sharp, A.L.; Huang, B.Z.; Tang, T.; Shen, E.; Melnick, E.R.; Venkatesh, A.K.; Kanter, M.H.; Gould, M.K. Implementation of the Canadian CT Head Rule and Its Association with Use of Computed Tomography Among Patients with Head Injury. Ann. Emerg. Med. 2018, 71, 54–63.e2.
- Galetta, K.M.; Liu, M.; Leong, D.F.; Ventura, R.E.; Galetta, S.L.; Balcer, L.J. The King-Devick test of rapid number naming for concussion detection: Meta-analysis and systematic review of the literature. Concussion 2015, 1, CNC8.
- Ashton, J.; Coyles, G.; Malone, J.J.; Roberts, J.W. Immediate effects of an acute bout of repeated soccer heading on cognitive performance. Sci. Med. Footb. 2021, 5, 181–187.
- Dessy, A.M.; Yuk, F.J.; Maniya, A.Y.; Gometz, A.; Rasouli, J.J.; Lovell, M.R.; Choudhri, T.F. Review of Assessment Scales for Diagnosing and Monitoring Sports-related Concussion. Cureus 2017, 9, e1922.
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M.; et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): Background and rationale. Br. J. Sports Med. 2017, 51, 848–850.
- Hobson, J. The Montreal cognitive assessment (MoCA). Occup. Med. 2015, 65, 764–765.
- Panwar, N.; Purohit, D.; Sinha, V.D.; Joshi, M. Evaluation of extent and pattern of neurocognitive functions in mild and moderate traumatic brain injury patients by using Montreal Cognitive Assessment (MoCA) score as a screening tool: An observational study from India. Asian J. Psychiatr. 2019, 41, 60–65.
- Stephenson, K.; Womble, M.N.; Eagle, S.; Collins, M.W.; Kontos, A.P.; Elbin, R.J. Symptom Provocation Following Post-concussion Computerized Neurocognitive Testing and Its Relationship to Other Clinical Measures of Concussion. Arch. Clin. Neuropsychol. 2023, 38, 548–556.
- Cromer, J.A.; Harel, B.T.; Yu, K.; Valadka, J.S.; Brunwin, J.W.; Crawford, C.D.; Mayes, L.C.; Maruff, P. Comparison of Cognitive Performance on the Cogstate Brief Battery When Taken In-Clinic, In-Group, and Unsupervised. Clin. Neuropsychol. 2015, 29, 542–558.
- Peiffer, A.J.; MacDonald, J.; Duerson, D.; Mitchell, G.; Hartwick, A.T.E.; McDaniel, C.E. The Influence of Binocular Vision Symptoms on Computerized Neurocognitive Testing of Adolescents with Concussion. Clin. Pediatr. 2020, 59, 961–969.
- Korley, F.K.; Jain, S.; Sun, X.; Puccio, A.M.; Yue, J.K.; Gardner, R.C.; Wang, K.K.W.; Okonkwo, D.O.; Yuh, E.L.; Mukherjee, P.; et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: An observational cohort study. Lancet Neurol. 2022, 21, 803–813.
- Biberthaler, P.; Musaelyan, K.; Krieg, S.; Meyer, B.; Stimmer, H.; Zapf, J.; von Matthey, F.; Chandran, R.; Marino, J.A.; Beligere, G.; et al. Evaluation of Acute Glial Fibrillary Acidic Protein and Ubiquitin C-Terminal Hydrolase-L1 Plasma Levels in Traumatic Brain Injury Patients with and without Intracranial Lesions. Neurotrauma Rep. 2021, 2, 617–625.
- Czeiter, E.; Amrein, K.; Gravesteijn, B.Y.; Lecky, F.; Menon, D.K.; Mondello, S.; Newcombe, V.F.J.; Richter, S.; Steyerberg, E.W.; Vyvere, T.V.; et al. Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine 2020, 56, 102785.
- Papa, L.; Ladde, J.G.; O’Brien, J.F.; Thundiyil, J.G.; Tesar, J.; Leech, S.; Cassidy, D.D.; Roa, J.; Hunter, C.; Miller, S.; et al. Evaluation of Glial and Neuronal Blood Biomarkers Compared with Clinical Decision Rules in Assessing the Need for Computed Tomography in Patients with Mild Traumatic Brain Injury. JAMA Netw. Open 2022, 5, e221302.
- Martin, C.L. i-STAT—Combining Chemistry and Haematology in PoCT. Clin. Biochem Rev. 2010, 31, 81–84.
- Zimmer, L.; McDade, C.; Beyhaghi, H.; Purser, M.; Textoris, J.; Krause, A.; Blanc, E.; Pavlov, V.; Earnshaw, S. Cost-Effectiveness of Blood-Based Brain Biomarkers for Screening Adults with Mild Traumatic Brain Injury in the French Health Care Setting. J. Neurotrauma 2023, 40, 706–719.
- Larcher, R.; Lottelier, M.; Badiou, S.; Dupuy, A.M.; Bargnoux, A.S.; Cristol, J.P. Analytical Performances of the Novel i-STAT Alinity Point-of-Care Analyzer. Diagnostics 2023, 13, 297.
- Seo, J.D.; Song, D.Y.; Nam, Y.; Li, C.; Kim, S.; Lee, J.H.; Lee, K.; Song, J.; Song, S.H. Evaluation of analytical performance of Alinity i system on 31 measurands. Pract. Lab. Med. 2020, 22, e00185.
- Asken, B.M.; Yang, Z.; Xu, H.; Weber, A.G.; Hayes, R.L.; Bauer, R.M.; DeKosky, S.T.; Jaffee, M.S.; Wang, K.K.W.; Clugston, J.R. Acute Effects of Sport-Related Concussion on Serum Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase L1, Total Tau, and Neurofilament Light Measured by a Multiplex Assay. J. Neurotrauma 2020, 37, 1537–1545.
- Tabor, J.; Penner, L.; Cooper, J.; Ghodsi, M.; Galarneau, J.M.; Fraser, D.D.; Emery, C.; Wellington, C.L.; Debert, C.T. Characterizing factors influencing baseline plasma biomarkers for sport-related concussion in youth. J. Neurotrauma 2023, 40, 1638–1650.
- Wang, K.K.W.; Kobeissy, F.H.; Shakkour, Z.; Tyndall, J.A. Thorough overview of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein as tandem biomarkers recently cleared by US Food and Drug Administration for the evaluation of intracranial injuries among patients with traumatic brain injury. Acute Med. Surg. 2021, 8, e622.
- Hicks, C.; Dhiman, A.; Barrymore, C.; Goswami, T. Traumatic Brain Injury Biomarkers, Simulations and Kinetics. Bioengineering 2022, 9, 612.
- Chen, Y.C.; Chen, Y.L.; Kuo, D.P.; Li, Y.T.; Chiang, Y.H.; Chang, J.J.; Tseng, S.H.; Chen, C.Y. Personalized Prediction of Postconcussive Working Memory Decline: A Feasibility Study. J. Pers. Med. 2022, 12, 196.
- Hier, D.B.; Obafemi-Ajayi, T.; Thimgan, M.S.; Olbricht, G.R.; Azizi, S.; Allen, B.; Hadi, B.A.; Wunsch, D.C., 2nd. Blood biomarkers for mild traumatic brain injury: A selective review of unresolved issues. Biomark. Res. 2021, 9, 70.
- Laverse, E.; Guo, T.; Zimmerman, K.; Foiani, M.S.; Velani, B.; Morrow, P.; Adejuwon, A.; Bamford, R.; Underwood, N.; George, J.; et al. Plasma glial fibrillary acidic protein and neurofilament light chain, but not tau, are biomarkers of sports-related mild traumatic brain injury. Brain Commun. 2020, 2, fcaa137.
- Coyle, H.L.; Bailey, N.W.; Ponsford, J.; Hoy, K.E. Recovery of clinical, cognitive and cortical activity measures following mild traumatic brain injury (mTBI): A longitudinal investigation. Cortex 2023, 165, 14–25.
- Marwaa, M.N.; Klakk Egebæk, H.; Dalgaard Guldager, J. Occupational and Physiotherapy modalities used to support interdisciplinary rehabilitation after concussion: A Scoping Review. J. Rehabil. Med. 2023, 55, jrm4512.
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166.
- Fried, E.; Balla, U.; Catalogna, M.; Kozer, E.; Oren-Amit, A.; Hadanny, A.; Efrati, S. Persistent post-concussive syndrome in children after mild traumatic brain injury is prevalent and vastly underdiagnosed. Sci. Rep. 2022, 12, 4364.
- Mannix, R.; Levy, R.; Zemek, R.; Yeates, K.O.; Arbogast, K.; Meehan, W.P.; Leddy, J.; Master, C.; Mayer, A.R.; Howell, D.R.; et al. Fluid Biomarkers of Pediatric Mild Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2020, 37, 2029–2044.
- Gardner, R.C.; Puccio, A.M.; Korley, F.K.; Wang, K.K.W.; Diaz-Arrastia, R.; Okonkwo, D.O.; Puffer, R.C.; Yuh, E.L.; Yue, J.K.; Sun, X.; et al. Effects of age and time since injury on traumatic brain injury blood biomarkers: A TRACK-TBI study. Brain Commun. 2022, 5, fcac316.
- Ward, M.D.; Weber, A.; Merrill, V.D.; Welch, R.D.; Bazarian, J.J.; Christenson, R.H. Predictive Performance of Traumatic Brain Injury Biomarkers in High-Risk Elderly Patients. J. Appl. Lab. Med. 2020, 5, 91–100.
- Pelkmans, W.; Shekari, M.; Brugulat-Serrat, A.; Sánchez-Benavides, G.; Minguillón, C.; Fauria, K.; Molinuevo, J.L.; Grau-Rivera, O.; González Escalante, A.; Kollmorgen, G.; et al. Astrocyte biomarkers GFAP and YKL-40 mediate early Alzheimer’s disease progression. Alzheimers Dement. 2023; Advance online publication.
- Lennon, M.J.; Brooker, H.; Creese, B.; Thayanandan, T.; Rigney, G.; Aarsland, D.; Hampshire, A.; Ballard, C.; Corbett, A.; Raymont, V. Lifetime Traumatic Brain Injury and Cognitive Domain Deficits in Late Life: The PROTECT-TBI Cohort Study. J. Neurotrauma 2023, 40, 1423–1435.
- Newcombe, V.F.J.; Ashton, N.J.; Posti, J.P.; Glocker, B.; Manktelow, A.; Chatfield, D.A.; Winzeck, S.; Needham, E.; Correia, M.M.; Williams, G.B.; et al. Post-acute blood biomarkers and disease progression in traumatic brain injury. Brain 2022, 145, 2064–2076.
- Breton M. Asken; Russell M. Bauer; Kevin M. Guskiewicz; Michael A. McCrea; Julianne D. Schmidt; Christopher C. Giza; Aliyah R. Snyder; Zachary M. Houck; Anthony P. Kontos; Thomas W. McAllister; et al. Immediate Removal From Activity After Sport-Related Concussion Is Associated With Shorter Clinical Recovery and Less Severe Symptoms in Collegiate Student-Athletes. Am. J. Sports Med. 2018, 46, 1465-1474, .
- Thomas R. Campbell; Nicholas Reilly; Martina Zamponi; Delaney Leathers; Peter A. Mollica; Julie Cavallario; Jessica C. Martinez; Salivary microRNA as a prospective tool for concussion diagnosis and management: A scoping review. Brain Inj. 2023, 37, 588-595.
- Hicks, S. D., Leddy, J., Lichak, B. P., Onks, C., Dretsch, M., Tennant, P., Haider, M. N., Olympia, R. P., Zuckerman, S. L., Loeffert, J., Loeffert, A. C., Monteith, C., & Master, C. L.; Defining Biological Phenotypes of Mild Traumatic Brain Injury Using Saliva MicroRNA Profiles.. J Neurotrauma 2022, 39, 923-934.
