Soot formation is an inevitable consequence of the combustion of carbonaceous fuels in environments rich in reducing agents. Efficient management of pollution in various contexts, such as industrial fires, vehicle engines, and similar applications, relies heavily on the subsequent oxidation of soot particles. Among the oxidizing agents employed for this purpose, oxygen, carbon dioxide, water vapor, and nitrogen dioxide have all demonstrated effectiveness.
- soot
- impact
- oxidation
- catalysts
1. Soot Formation and Methodology for Soot Oxidation
A few years ago, diesel engines gained worldwide fame owing to their unique features, such as low fuel consumption, long range, and greater thermal efficiency, compared with other engines [1][2]. The other side of the picture is also quite harsh given diesel engine exhaust emissions, which create a great threat for us on this planet [3][4][5]. The composition of their exhaust emanations is a combination of different gases, vapors, particulate matter (soot), liquid aerosols, nitrogen, water, CO, NOx, SOx, and polycyclic aromatic hydrocarbons (PAHs), and they make our world alarmingly polluted with air pollution [6][7][8]. The overall composition of diesel exhausts and their threats to human life and the environment are described in Table 1 [9][10][11][12].
| Pollutants | Concentration | Threats |
|---|---|---|
| Soot | 20–200 mg/m3 | Eyes problems, cancer, asthma, skin infections, lung damage, heart issues |
| NOx | 30–1000 ppm | Chest pain, respiratory and lungs problems, cough |
| SOx | Proportional to fuel S content | Acid rain, skin problems |
| CO2 | 2–12 vol% | Green house effect, acid rain, lung disease |
| CO | 100–1000 ppm | Hpertension, head pressure, lung disease |
| HC | 50–500 ppm | Eyes irritation, lungs issues, respiratory problems |
| PAH | 0.3 mg/mil | Kindney and liver damage |
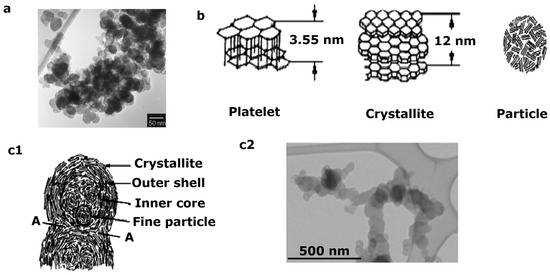
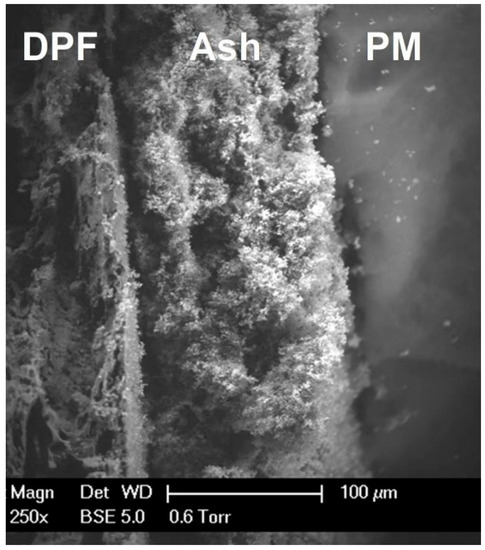
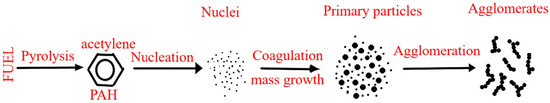
2. Effect on Health and the Environment
3. Diversity in Catalysts for Removal of Soot
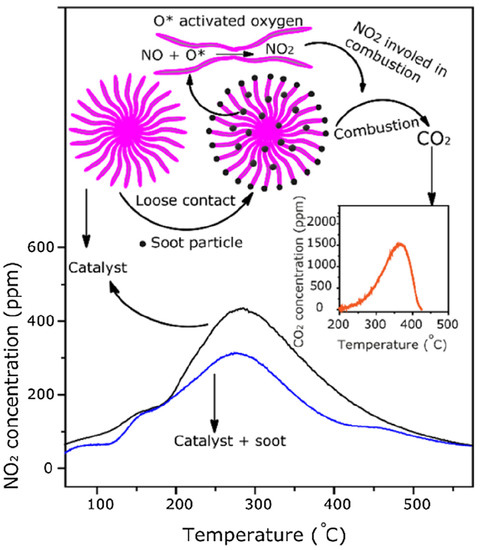
4. Ceria-Based Mixed Metal Oxides
Ceria (CeO2) is of most significant importance as a component of three-way catalysts (TWCs) given its storage capacity (OSC) for oxygen [78]. It has attained a significant rank among the metal oxides that have been extensively studied to date [79][80]. The research direction proposed by Trovarelli has opened a new door for ceria-based catalysts, indicating their potential in theoretical and practical applications as well as providing structural insights for their derived catalysts. Meanwhile, they function to support and boost the catalytic performances of metal catalysts [81]. The effects of the nanometric sizes and morphologies of ceria-based catalysts have been studied since the last decade, and various studies have reported on their synthesis pathways, chemical properties, geometries, characteristics, and catalytic performance in the oxidation of CO to date [82]. Recently, a correlation has been reported for redox properties between surface properties and the crystal morphology of ceria-based cubes, polyhedrons, and rods. Observations indicated that face reconstruction, size, and nanomorphology influence their performance, selectivity and stability [83]. In the ceria cubic structure, the fcc group, which is regarded as a stable surface plane, shows a lower coordination number compared to its bulk with divergent terminating structures on surfaces, including repetitive O-Ce-O interlayers, both elements Ce and O, and a O-Ce-O-Ce echoing unit. However, in thermally controlled systems, stable surfaces are normally generated during crystal growth and finally develop specific nanoshapes [84].
Remarkably, every stable plane displays various reduction features. The redox process of Ce4+ to Ce3+ produces vacancies for oxygen that play a vital role in oxygen packing and oxidation reactions. There is no theoretical basis; the growth plans follow the order of reactivity for oxygen vacancy defect formation, providing the basis for experimental work to assess the relationship between the catalytic performance and nanocrystal morphology of ceria [85]. The oxygen vacancies and surface chemistry strongly depend on the nanometric size of particles, and these factors are strongly enhanced when the particle size is less than 10 nm. Oxygen vacancy creation modeling investigations focused on size revealed that their energy is governed by the position of the oxygen atom lattice; for nanoparticles (NPs) with a size of 2–4 nm, its value approaches the minimum level [86].
The catalytic performance of the nanorods was observed to be associated with loosely bound oxygen. The nanorods’ performances were lower than those of nanowires, regardless of the fact that nanorods and nanowires exhibit predominantly reactive planes; this could be attributed to a higher concentration of surface-active planes [87]. Hierarchically, mesoporous ceria is prepared using diatom templates, which have greater Ce3+ content, a high specific surface area (SSA) (78 m2 g1), facile reducibility, a higher number of oxygen vacancies, and enhanced CO oxidation compared with bulk ceria. Moreover, ultrasound synthesis was reported to form nanoflowers, nanospheres, nanorods, and nanoribbons of ceria nanostructures (size ~5 nm) [88]. This synthesis was performed in a single step using various kinds of ionic liquids. The shape and structure of the final product depend on how it was heated. For example, under [C4mim][Tf2N], the ionothermal fabrication method produced flower and nanorod shapes, while the ultrasound method produced nanospheres. Nanoshape activity order followed the order of the SSAs; however, this order was not found to be proportional to them, indicating that oxygen vacancies as well as structural defects play crucial roles. Sonochemistry under [C4mim][Tf2N] generates nanospheres with the best performance. This is because the nanospheres have a large SSA, a mesoporous structure, a higher number of surface oxygen vacancies, and small particle size [89].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28196884
References
- Pierce, D.; Haynes, A.; Hughes, J.; Graves, R.; Maziasz, P.; Muralidharan, G.; Shyam, A.; Wang, B.; England, R.; Daniel, C. High temperature materials for heavy duty diesel engines: Historical and future trends. Prog. Mater. Sci. 2019, 103, 109–179.
- Prasad, R.; Singh, S.V. A review on catalytic oxidation of soot emitted from diesel fuelled engines. J. Environ. Chem. Eng. 2020, 8, 103945.
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions of diesel engines operating under transient conditions with biodiesel fuel blends. Prog. Energy Combust. Sci. 2012, 38, 691–715.
- He, L.; Zhang, Y.; Zang, Y.; Liu, C.; Wang, W.; Han, R.; Ji, N.; Zhang, S.; Liu, Q. Promotion of A-site Ag-doped perovskites for the catalytic oxidation of soot: Synergistic catalytic effect of dual active sites. ACS Catal. 2021, 11, 14224–14236.
- Mizushima, N.; Kawano, D.; Ishii, H.; Takada, Y.; Sato, S. Evaluation of Real-World Emissions from Heavy-Duty Diesel Vehicle Fueled with FAME, HVO and BTL Using PEMS; 0148-7191; SAE Technical Paper: Warrendale, PA, USA, 2014.
- Sethi, C.; Patnaik, P.; Thatoi, D.; Acharya, S. Performance, Combustion & Emission Analysis on Diesel Engine Utilizing Diethyl Ether as a Fuel Additives. TEST Eng. Manag. 2020, 82, 2391–2408.
- Subramanian, K.; Gnanam, A.; Damodharan, D.; Prasanna, N.; Mukilarasan, N. Emission Control and Reduction in Fuel Consumption of Two-Stroke Si Engine Using Nano-Fragment as a Catalyst; AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2020.
- Mahla, S.K.; Ardebili, S.M.S.; Sharma, H.; Dhir, A.; Goga, G.; Solmaz, H. Determination and utilization of optimal diesel/n-butanol/biogas derivation for small utility dual fuel diesel engine. Fuel 2021, 289, 119913.
- Prasad, R.; Bella, V.R. A review on diesel soot emission, its effect and control. Bull. Chem. React. Eng. Catal. 2010, 5, 69–86.
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238.
- Zhang, Z.-H.; Khlystov, A.; Norford, L.K.; Tan, Z.-K.; Balasubramanian, R. Characterization of traffic-related ambient fine particulate matter (PM2.5) in an Asian city: Environmental and health implications. Atmos. Environ. 2017, 161, 132–143.
- Dhal, G.C.; Mohan, D.; Prasad, R. Preparation and application of effective different catalysts for simultaneous control of diesel soot and NOX emissions: An overview. Catal. Sci. Technol. 2017, 7, 1803–1825.
- Zhao, F.; Yang, W.; Yu, W. A progress review of practical soot modelling development in diesel engine combustion. J. Traffic Transp. Eng. 2020, 7, 269–281.
- Zygogianni, A.; Syrigou, M.; Konstandopoulos, A.G.; Kostoglou, M. Oxidative reactivity of particulate samples from different diesel combustion systems and its relation to structural and spectral characteristics of soot. Emiss. Control Sci. Technol. 2019, 5, 99–123.
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102.
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B Environ. 2016, 197, 105–115.
- Liu, Y.; Zhang, X.; Lyu, G.; Qiao, Y.; Zhang, W.; Song, C. Effect of the oxidation-induced fragmentation of primary particles on soot oxidation reactivity. Combust. Flame 2022, 240, 112026.
- Yang, W.; Wang, Y.; Wang, H.; Zhang, Y.; Peng, Y.; Li, J. Water accelerates and directly participates soot oxidation: An isotopic study. Appl. Catal. B Environ. 2022, 302, 120837.
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11.
- Bagi, S.; Sharma, V.; Patel, M.; Aswath, P.B. Effects of diesel soot composition and accumulated vehicle mileage on soot oxidation characteristics. Energy Fuels 2016, 30, 8479–8490.
- Wei, J.; Fan, C.; Zhuang, Y.; Fu, Z.; Guan, Z.; Li, H.; Li, D.; Qian, Y. Diesel soot combustion over ceria catalyst: Evolution of functional groups on soot surfaces. Fuel 2023, 338, 127391.
- Xi, J.; Zhong, B.J. Soot in diesel combustion systems. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2006, 29, 665–673.
- Altenhoff, M.; Aßmann, S.; Teige, C.; Huber, F.J.; Will, S. An optimized evaluation strategy for a comprehensive morphological soot nanoparticle aggregate characterization by electron microscopy. J. Aerosol Sci. 2020, 139, 105470.
- Kennedy, I.M. Models of soot formation and oxidation. Prog. Energy Combust. Sci. 1997, 23, 95–132.
- De Falco, G.; Picca, F.; Commodo, M.; Minutolo, P. Probing soot structure and electronic properties by optical spectroscopy. Fuel 2020, 259, 116244.
- Mathis, U.; Mohr, M.; Kaegi, R.; Bertola, A.; Boulouchos, K. Influence of diesel engine combustion parameters on primary soot particle diameter. Env. Sci Technol 2005, 39, 1887–1892.
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx–CeO2–Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290.
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608.
- Siegmann, K.; Siegmann, H.C. Molecular Precursor of Soot and Quantification of the Associated Health Risk. In Current Problems in Condensed Matter; Morán-López, J.L., Ed.; Springer: Boston, MA, USA, 1998; pp. 143–160.
- Richter, H.; Granata, S.; Green, W.H.; Howard, J.B. Detailed modeling of PAH and soot formation in a laminar premixed benzene/oxygen/argon low-pressure flame. Proc. Combust. Inst. 2005, 30, 1397–1405.
- Tree, D.R.; Svensson, K.I. Soot processes in compression ignition engines. Prog. Energy Combust. Sci. 2007, 33, 272–309.
- Jaramillo, I.C.; Gaddam, C.K.; Vander Wal, R.L.; Huang, C.-H.; Levinthal, J.D.; Lighty, J.S. Soot oxidation kinetics under pressurized conditions. Combust. Flame 2014, 161, 2951–2965.
- Gao, Y.; Jin, B.; Xi, Z.; Li, Z.; Wu, X.; Ran, R.; Si, Z.; Weng, D. Nitration Poisoning of Silver on Al2O3 for the Catalytic Oxidation of Soot in NO x-Containing Atmospheres. Ind. Eng. Chem. Res. 2022, 61, 15893–15899.
- Yang, F. Under the Dome: “Chinese” smog as a viral media event. Crit. Stud. Media Commun. 2016, 33, 232–244.
- Kerr, R.A. Soot is Warming the World Even More than Thought; American Association for the Advancement of Science: Washington, DC, USA, 2013.
- Tollefson, J. Soot a major contributor to climate change. Nature 2013, 15, 15.
- Lohmann, U.; Friebel, F.; Kanji, Z.A.; Mahrt, F.; Mensah, A.A.; Neubauer, D. Future warming exacerbated by aged-soot effect on cloud formation. Nat. Geosci. 2020, 13, 674–680.
- Manosh, P. Soot: Sources, Formation and Health Effects; Nova Science Publishers: New York, NY, USA, 2012.
- Liu, S.; Wu, X.; Weng, D.; Li, M.; Lee, H.-R. Combined promoting effects of platinum and MnOx-CeO2 supported on alumina on NOx-assisted soot oxidation: Thermal stability and sulfur resistance. Chem. Eng. J. 2012, 203, 25–35.
- Lisi, L.; Landi, G.; Di Sarli, V. The issue of soot-catalyst contact in regeneration of catalytic diesel particulate filters: A critical review. Catalysts 2020, 10, 1307.
- Chi, H.; Zhang, P.; Xiong, J.; Wei, Y.; Li, Y.; Zhao, Z.; Liu, J.; Jiao, J. Single-crystalline α-MnO2 catalysts with tailored exposed crystal facets for boosting catalytic soot oxidation: The crystal facet-dependent activity. Appl. Surf. Sci. 2023, 608, 155116.
- Hu, C.; Dai, P.; Chen, Z.; Zhang, H. Property and Reactivity Relationships of Co3O4 with Diverse Nanostructures for Soot Oxidation. ACS Omega 2022, 7, 44116–44123.
- Jampaiah, D.; Velisoju, V.K.; Venkataswamy, P.; Coyle, V.E.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Nanowire morphology of mono-and bidoped α-MnO2 catalysts for remarkable enhancement in soot oxidation. ACS Appl. Mater. Interfaces 2017, 9, 32652–32666.
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. Effect of the morphological and surface properties of CeO2-based catalysts on the soot oxidation activity. Chem. Eng. J. 2015, 278, 190–198.
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. The role of metal-support interaction in Ag/CeO2 catalysts for CO and soot oxidation. Appl. Catal. B Environ. 2020, 260, 118148.
- Wang, B.; Wang, Z.; Ai, L.; Liu, W.; Li, Q.; Wang, X.; Wang, L. High performance of K-supported Pr2Sn2O7 pyrochlore catalysts for soot oxidation. Fuel 2022, 317, 123467.
- Uppara, H.P.; Singh, S.K.; Labhsetwar, N.K.; Murari, M.S.; Dasari, H. The decisive factor of hollow spherical network morphology of Nd1-xCexCo1-yCuyO3±δ perovskites towards soot oxidation. Chem. Pap. 2022, 76, 3771–3787.
- Li, Y.; Guo, H.; Xiong, J.; Ma, Y.; Li, X.; Zhang, P.; Zhang, S.; Wei, Y. The Catalyst of Ruthenium Nanoparticles Decorated Silicalite-1 Zeolite for Boosting Catalytic Soot Oxidation. Catalysts 2023, 13, 1167.
- Voskanyan, A.A.; Chan, K.Y. Scalable synthesis of three-dimensional meso/macroporous NiO with uniform ultralarge randomly packed mesopores and high catalytic activity for soot oxidation. ACS Appl. Nano Mater. 2018, 1, 556–563.
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751.
- Shuang, L.; Xiaodong, W.; Duan, W.; Rui, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590.
- Lou, D.; Xiang, B.; Zhang, Y.; Fang, L.; Tan, P.; Hu, Z. Study on the Catalytic Characteristics of Precious Metal Catalysts with Different Pt/Pd Ratios for Soot Combustion. ACS Omega 2023, 8, 20834–20844.
- Khan, A.U.; Ullah, S.; Yuan, Q.; Ali, S.; Ahmad, A.; Khan, Z.U.H.; Rahman, A.U. In situ fabrication of Au–CoFe2O4: An efficient catalyst for soot oxidation. Appl. Nanosci. 2020, 10, 3901–3910.
- Oh, D.-K.; Lee, Y.-J.; Lee, K.-Y.; Park, J.-S. Nitrogen Monoxide and Soot Oxidation in Diesel Emissions with Platinum–Tungsten/Titanium Dioxide Catalysts: Tungsten Loading Effect. Catalysts 2020, 10, 1283.
- Mukherjee, D.; Reddy, B.M. Noble metal-free CeO2-based mixed oxides for CO and soot oxidation. Catal. Today 2018, 309, 227–235.
- Martinovic, F.; Galletti, C.; Bensaid, S.; Pirone, R.; Deorsola, F.A. Soot oxidation in low-O2 and O2-free environments by lanthanum-based perovskites: Structural changes and the effect of Ag doping. Catal. Sci. Technol. 2022, 12, 5453–5464.
- Mishra, A.; Prasad, R. Preparation and application of perovskite catalysts for diesel soot emissions control: An overview. Catal. Rev. 2014, 56, 57–81.
- Zhang, R.; Alamdari, H.; Kaliaguine, S. Fe-based perovskites substituted by copper and palladium for NO+ CO reaction. J. Catal. 2006, 242, 241–253.
- Hernández, W.; Tsampas, M.; Zhao, C.; Boreave, A.; Bosselet, F.; Vernoux, P. La/Sr-based perovskites as soot oxidation catalysts for Gasoline Particulate Filters. Catal. Today 2015, 258, 525–534.
- Shao, W.; Wang, Z.; Zhang, X.; Wang, L.; Ma, Z.; Li, Q.; Zhang, Z. Promotion effects of cesium on perovskite oxides for catalytic soot combustion. Catal. Lett. 2016, 146, 1397–1407.
- Jiménez, R.; Zamora, R.; Pecchi, G.; García, X.; Gordon, A. Effect of Ca-substitution in La1—xCaxFeO3 perovskites on the catalytic activity for soot combustion. Fuel Process. Technol. 2010, 91, 546–549.
- Torregrosa-Rivero, V.; Sanchez-Adsuar, M.-S.; Illan-Gomez, M.-J. Analyzing the role of copper in the soot oxidation performance of BaMnO3-perovskite-based catalyst obtained by modified sol-gel synthesis. Fuel 2022, 328, 125258.
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002.
- Pecchi, G.; Dinamarca, R.; Campos, C.M.; Garcia, X.; Jimenez, R.; Fierro, J.L. Soot oxidation on silver-substituted LaMn0.9Co0.1O3 perovskites. Ind. Eng. Chem. Res. 2014, 53, 10090–10096.
- Wang, M.; Han, Z.; Liu, Y.; Gao, C.; Pan, X.; Zhou, S. The influence of partial substitution of Ce with K in CeMO3 (M= Mn, Fe, Co, Ni, Cu) perovskite catalysts on soot combustion performance. J. Environ. Chem. Eng. 2023, 11, 110850.
- Mishra, A.; Prasad, R. Synthesis and performance of transition metal based perovskite catalysts for diesel soot oxidation. Bull. Chem. React. Eng. Catal. 2017, 12, 469–477.
- Fan, Q.; Zhang, S.; Sun, L.; Dong, X.; Zhang, L.; Shan, W.; Zhu, Z. Catalytic oxidation of diesel soot particulates over Ag/LaCoO3 perovskite oxides in air and NOx. Chin. J. Catal. 2016, 37, 428–435.
- Feng, N.; Wu, Y.; Meng, J.; Chen, C.; Wang, L.; Wan, H.; Guan, G. Catalytic combustion of soot over Ce and Co substituted three-dimensionally ordered macroporous La 1–x CexFe1–yCoyO3 perovskite catalysts. RSC Adv. 2015, 5, 91609–91618.
- Luo, J.; Zhu, X.; Wu, H.; Zhou, Z.; Chen, G.; Yang, G. Soot oxidation over V/ZSM-5 catalysts in a dielectric barrier discharge (DBD) reactor: Performance enhancement by transition metal (Mn, Co and Fe) doping. Catal. Today 2023, 419, 114139.
- Tao, F.; Golovitchev, V.I.; Chomiak, J. A phenomenological model for the prediction of soot formation in diesel spray combustion. Combust. Flame 2004, 136, 270–282.
- Chang, H.; Bjørgum, E.; Mihai, O.; Yang, J.; Lein, H.L.; Grande, T.; Raaen, S.; Zhu, Y.-A.; Holmen, A.; Chen, D. Effects of oxygen mobility in La–Fe-based perovskites on the catalytic activity and selectivity of methane oxidation. ACS Catal. 2020, 10, 3707–3719.
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965.
- Bukhtiyarova, M. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506.
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater. Today 2016, 19, 213–226.
- Ali, S.; Wu, X.; Zuhra, Z.; Ma, Y.; Abbas, Y.; Jin, B.; Ran, R.; Weng, D. Cu-Mn-Ce mixed oxides catalysts for soot oxidation and their mechanistic chemistry. Appl. Surf. Sci. 2020, 512, 145602.
- Wang, M.; Zhang, Y.; Yu, Y.; Shan, W.; He, H. Insight into the better performance of Co than Pt on Ce-Sn catalyst for soot oxidation. Fuel 2023, 346, 128379.
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of Mixed Metal Oxides in Catalysis Science—Versatile Applications in Organic Synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125.
- Shukla, M.; Balyan, Y.; Kumar, A.; Bhaskar, T.; Dhar, A. Catalytic oxidation of soot by CeO2–ZrO2 catalysts: Role of Zr. Mater. Chem. Phys. 2022, 286, 126161.
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520.
- Wang, L.; Zhao, N.; Yin, X.; Wang, W.; Zhao, Y.; Zheng, Z.; Li, S.; Wang, J.; Chen, Y. Highlights on the key roles of interfaces between CeO2-based oxide and perovskite (LaMnO3/LaFeO3) in creating active oxygen species for soot oxidation. Fuel 2024, 356, 129444.
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475.
- Tang, W.-X.; Gao, P.-X. Nanostructured cerium oxide: Preparation, characterization, and application in energy and environmental catalysis. Mrs Commun. 2016, 6, 311–329.
- Wu, K.; Sun, L.-D.; Yan, C.-H. Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501.
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? Acs Catal. 2017, 7, 4716–4735.
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-Controlled Ceria-based Nanostructures for Catalysis Applications. Chemsuschem 2013, 6, 1821–1833.
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183.
- Shan, W.; Guo, H.; Liu, C.; Wang, X. Controllable preparation of CeO2 nanostructure materials and their catalytic activity. J. Rare Earths 2012, 30, 665–669.
- Qian, J.; Chen, Z.; Liu, C.; Wang, F.; Zhang, Y.; Wang, M. Biotemplated fabrication of hierarchical mesoporous CeO2 derived from diatom and its application for catalytic oxidation of CO. Chin. Sci. Bull. 2014, 59, 3260–3265.
- Alammar, T.; Noei, H.; Wang, Y.; Gruener, W.; Mudring, A.-V. Ionic Liquid-Assisted Sonochemical Preparation of CeO2 Nanoparticles for CO Oxidation. Acs Sustain. Chem. Eng. 2015, 3, 42–54.
