Graft-versus-host disease (GVHD) is a complication of hematopoietic stem cell transplantation (HSCT). GVHD may also develop following solid transplants or blood transfusions if white blood cells are transferred. GVHD affects multiple organs, including the oral tissues.
- graft-versus-host disease
- oral manifestations
- dental treatment
1. Introduction
-
Graft containing supply of immunocompetent cells [4]
-
Immunological dissimilarity between host and donor
-
Immunosuppressed host.
1.1. How It Works: Allo-HSCT
-
Resolution of the disease
-
Allogeneic stem cell engraftment (production of the necessary space for the infusion)
-
Bone marrow restoration by the infused cells after a period of aplasia (which takes about 100 days)
-
Elimination of remaining diseased cells thanks to the graft-versus-tumor effect (GvT): allogeneically transplanted human hematopoietic cells can eliminate residual tumor cells in the recipient by an immune-mediated mechanism [3].
-
Bone marrow (BM).
-
Peripheral blood stem cells (PBSC), which is the most innovative and most used technique to date.
-
Umbilical cord blood, which, however, does not provide a quantity of stem cells sufficient to treat an adult.
1.2. Pre-HSCT Dentistry Videat
|
Pathology |
Pathological State |
Treatment Options |
|---|---|---|
|
Carious pathology |
|
Restorative (if sufficient timing or no treatment), Pulpectomy |
|
Apical paradentitis |
|
Extraction or root canal therapy (depending on available time frame) Extraction or root canal therapy if radiotransparency > 5 mm No treatment for radiotransparency < 5 mm |
|
Periodontal disease |
|
Extraction Extraction Renew dental hygiene instructions and perform scaling maneuvers |
|
Partially erupted third molars |
|
Extraction No treatment |
Epidemiology
1.3. Acute and Chronic GVHD
-
Persistent, if characterized by the persistence of signs and symptoms of classic aGvHD initiated before day 100 post-transplant;
-
Recurrent, if it arises as a classic form that resolves but recurs after day 100 post-transplant;
-
De novo, if it occurs after day 100 post-transplant without any signs or symptoms in the previous time frame.
1.4. Oral GVHD
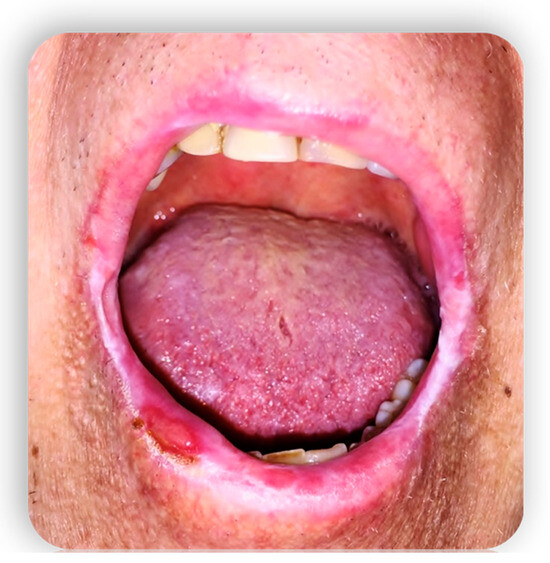
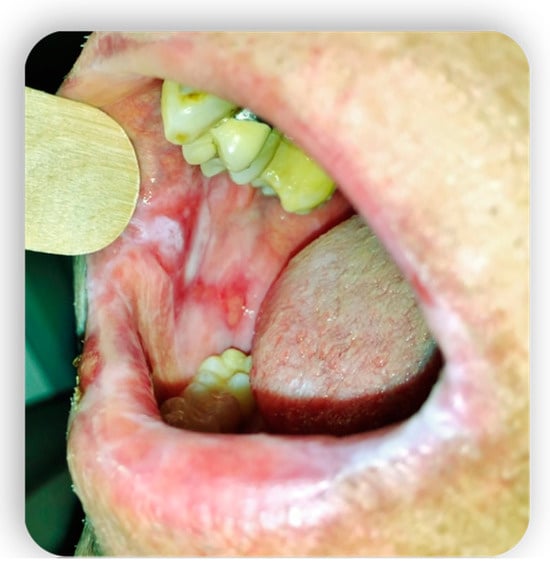
2. Systemic Treatment of Oral Lesions
3. Topical Treatment of Oral Lesions
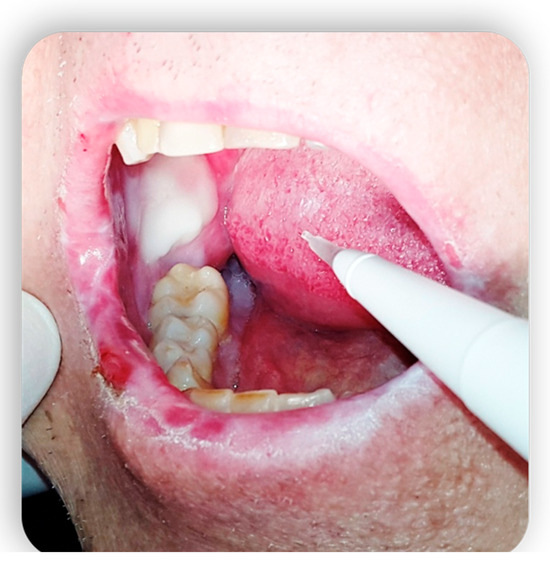
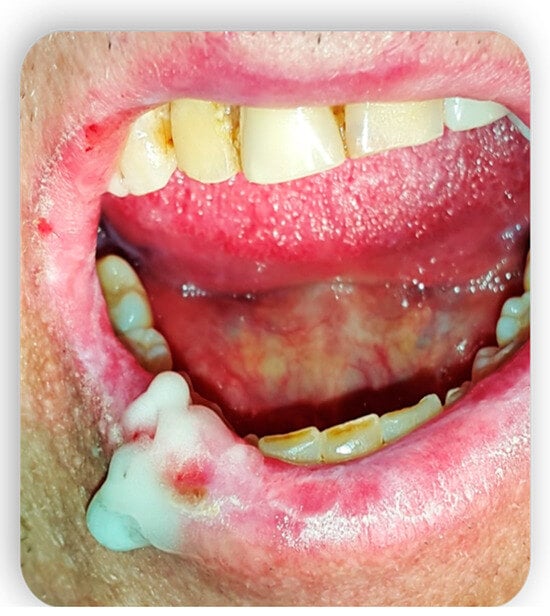
4. GvHD and Implantology: Case Reports in the Literature
This entry is adapted from the peer-reviewed paper 10.3390/medicina59111937
References
- Lee, S.J. Classification systems for chronic graft-versus-host disease. Blood 2017, 129, 30–37.
- Arora, M.; Nagaraj, S.; Witte, J.; DeFor, T.E.; MacMillan, M.; Burns, L.J.; Weisdorf, D.J. New classification of chronic GVHD: Added clarity from the consensus diagnoses. Bone Marrow Transpl. 2009, 43, 149–153.
- Flowers, M.E.D.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.-P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219.
- Pavletic, S.Z.; Martin, P.; Lee, S.J.; Mitchell, S.; Jacobsohn, D.; Cowen, E.W.; Turner, M.L.; Akpek, G.; Gilman, A.; McDonald, G.; et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol. Blood Marrow Transplant. 2006, 12, 252–266.
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1.
- Ion, D.; Stevenson, K.; Woo, S.-B.; Ho, V.T.; Soiffer, R.; Antin, J.H.; Treister, N.S. Characterization of oral involvement in acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 1717–1721.
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579.
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431.
- Yuan, A.; Chai, X.; Martins, F.; Arai, S.; Arora, M.; Correa, M.E.; Pidala, J.; Cutler, C.S.; Lee, S.J.; Treister, N.S. Oral chronic GVHD outcomes and resource utilization: A subanalysis from the chronic GVHD consortium. Oral. Dis. 2016, 22, 235–240.
- Brunstein, C.G.; Fuchs, E.J.; Carter, S.L.; Karanes, C.; Costa, L.J.; Wu, J.; Devine, S.M.; Wingard, J.R.; Aljitawi, O.S.; Cutler, C.S.; et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011, 118, 282–288.
- Al-Hashimi, I.; Schifter, M.; Lockhart, P.B.; Wray, D.; Brennan, M.; Migliorati, C.A.; Axéll, T.; Bruce, A.J.; Carpenter, W.; Eisenberg, E.; et al. Oral lichen planus and oral lichenoid lesions: Diagnostic and therapeutic considerations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103 (Suppl. S25), e1–e12.
- Elad, S.; Zadik, Y.; Yarom, N. Oral Complications of Nonsurgical Cancer Therapies. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2017, 25, 133–147.
- Zadik, Y. Restricted mouth opening in chronic graft-versus-host disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 201–202.
- Bertelli, L.; Di Nardo, G.; Zama, D.; Bardasi, G.; Morello, W.; Masetti, R.; Belotti, T.; Forchielli, M.L.; Prete, A.; Pession, A. A New Formulation of an Old Drug: A Potential New Therapy in the Management of Oral cGvHD. J. Pediatr. Hematol. Oncol. 2016, 38, e295–e297.
- Gomes, C.B.F.; Treister, N.S.; Miller, B.; Armand, P.; Friedland, B. Pulp Obliteration in a Patient with Sclerodermatous Chronic Graft-versus-Host Disease. J. Endod. 2016, 42, 678–680.
- Zadik, Y.; Elad, S.; Shapira, A.; Shapira, M.Y. Treatment of oral mucosal manifestations of chronic graft-versus-host disease: Dexamethasone vs. budesonide. Expert. Opin. Pharmacother. 2017, 18, 235–242.
- Schubert, M.M. Correa MEP Oral graft-versus-host disease. Dent. Clin. N. Am. 2008, 52, 79–109.
- Ramachandran, V.; Kolli, S.S.; Strowd, L.C. Review of Graft-Versus-Host Disease. Dermatol. Clin. 2019, 37, 569–582.
- Fall-Dickson, J.M.; Pavletic, S.Z.; Mays, J.W.; Schubert, M.M. Oral Complications of Chronic Graft-Versus-Host Disease. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz007.
- Jiang, H.; Fu, D.; Bidgoli, A.; Paczesny, S. T Cell Subsets in Graft Versus Host Disease and Graft Versus Tumor. Front. Immunol. 2021, 12, 761448.
- Janowiak-Majeranowska, A.; Osowski, J.; Mikaszewski, B.; Majeranowski, A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers 2022, 14, 2175.
- Bleakley, M.; Sehgal, A.; Seropian, S.; Biernacki, M.A.; Krakow, E.F.; Dahlberg, A.; Persinger, H.; Hilzinger, B.; Martin, P.J.; Carpenter, P.A.; et al. Naive T-Cell Depletion to Prevent Chronic Graft-Versus-Host Disease. J. Clin. Oncol. 2022, 40, 1174–1185.
- Bazinet, A.; Popradi, G. A General Practitioner’s Guide to Hematopoietic Stem-cell Transplantation. Curr. Oncol. 2019, 26, 187–191.
- Treister, N.; Li, S.; Lerman, M.A.; Lee, S.; Soiffer, R. Narrow-band UVB phototherapy for management of oral chronic graft-versus-host disease. Photodermatol. Photoimmunol. Photomed. 2015, 31, 75–82.
- Miranda, M.; Gianfreda, F.; Rosa, A.; Fiorillo, L.; Cervino, G.; Cicciù, M.; Bollero, P. Treatment of Oral Mucositis Using Platelet-Rich-Fibrin: A Retrospective Study on Oncological Patients. J. Craniofac. Surg. 2023, 34, 1527–1529.
- Sánchez, A.R.; Sheridan, P.J.; Rogers, R.S. Successful treatment of oral lichen planus-like chronic graft-versus-host disease with topical tacrolimus: A case report. J. Periodontol. 2004, 75, 613–619.
- Dean, D.; Sroussi, H. Oral Chronic Graft-Versus-Host Disease. Front. Oral Health 2022, 3, 903154.
- Brown, R.S.; Edwards, D.; Walsh-Chocolaad, T.; Childs, R.W. Topical tacrolimus with custom trays in the treatment of severe oral chronic graft-versus-host disease refractory to a potent topical steroid therapy: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e26–e30.
- Vogelsang, G.B.; Wolff, D.; Altomonte, V.; Farmer, E.; Morison, W.L.; Corio, R.; Horn, T. Treatment of chronic graft-versus-host disease with ultraviolet irradiation and psoralen (PUVA). Bone Marrow Transplant. 1996, 17, 1061–1067.
- Curtis, J.R.; Yamaoka, K.; Chen, Y.-H.; Bhatt, D.L.; Gunay, L.M.; Sugiyama, N.; Connell, C.A.; Wang, C.; Wu, J.; Menon, S.; et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL Surveillance trial. Ann. Rheum. Dis. 2023, 82, 331–343.
- Etebarian, A.; Mirshamsi, H.; Sadeghi, H.S.; Hemmati, F. Oral rehabilitation in a patient with sclerotic-phenotype chronic graft versus host disease: A case report. Quintessence Int. 2019, 50, 208–213.
- Castellarin, P.; Stevenson, K.; Biasotto, M.; Yuan, A.; Woo, S.-B.; Treister, N.S. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2012, 18, 1573–1579.
- Bollero, P.; Franco, R.; Gianfreda, F.; Gualtieri, P.; Miranda, M.; Barlattani, A. Epidemiology, Etiopathogenesis, Treatment and Prognosis of Oral Thermal Burns from Food and Drinks. Dent. Hypotheses 2019, 10, 80.
- Thi, H.N.; Manh, C.P.; Tuan, L.T.; Le Thi, L.A.; Thanh, N.N.; Vilaiyuk, S. Tumor-Induced Osteomalacia Associated with a Maxillary Tumor in Children: A Case Report and Review of the Literature. J. Clin. Res. Pediatr. Endocrinol. 2022.
