For several decades now, researchers have been trying to answer the demand of clinical oncologists to create an ideal preclinical model of head and neck squamous cell carcinoma (HNSCC) that is accessible, reproducible, and relevant. Over the past years, the development of cellular technologies has naturally allowed us to move from primitive short-lived primary 2D cell cultures to complex patient-derived 3D models that reproduce the cellular composition, architecture, mutational, or viral load of native tumor tissue. Depending on the tasks and capabilities, a scientific laboratory can choose from several types of models: primary cell cultures, immortalized cell lines, spheroids or heterospheroids, tissue engineering models, bioprinted models, organoids, tumor explants, and histocultures. HNSCC in vitro models make it possible to screen agents with potential antitumor activity, study the contribution of the tumor microenvironment to its progression and metastasis, determine the prognostic significance of individual biomarkers (including using genetic engineering methods), study the effect of viral infection on the pathogenesis of the disease, and adjust treatment tactics for a specific patient or groups of patients. Promising experimental results have created a scientific basis for the registration of several clinical studies using HNSCC in vitro models.
- head and neck squamous cell carcinoma
- in vitro models
- 3D models
- viral infection
- personalized medicine
1. Transition from 2D to 3D HNSCC In Vitro Models
|
Cell Line |
Tissue |
Tumor Source |
Sex |
Karyotype |
Mutant Genes |
|
A-253 |
submaxillary salivary gland |
primary |
male |
near triploid with at least 6 markers |
CDKN2A KDMC5 TP53 |
|
SCC-9 |
Tongue |
primary |
male |
n.d. |
CDKN2A TP53 |
|
SCC-15 |
Tongue |
primary |
male |
n.d. |
TP53 |
|
SCC-25 |
Tongue |
primary |
male |
possible loss of Y chromosome |
CDKN2A TP53 |
|
FaDu |
hypopharynx |
primary |
male |
hypodiploid to hypertriploid with modal number = 64 |
CDKN2A SMAD4 TP53 |
|
Detroit 562 |
Pharynx |
metastasis (pleural effusion) |
female |
modal number = 64; range = 58 to 128 |
CDKN2A PIK3CA TP53 |
2. Three-Dimensional In Vitro Cell Models of HNSCC: Main Types and Methods of Production
2.1. Spheroids
- (1)
- (2)
- (3)
-
ensure continuous mixing of the cell suspension, preventing cells from settling and coming into contact with the substrate (agitation-based method, magnetic levitation) [53].
- (1)
-
part of the material may be initially contaminated with bacteria or fungi;
- (2)
-
during the cultivation process, epithelial cells can be replaced by more rapidly proliferating stromal cells;
- (3)
-
the rate of cell proliferation and the efficiency of spheroid formation decreases with an increase in the number of passages performed;
- (4)
|
HNSCC Cell Line (Organ) |
Additional Cellular Component |
Ratio |
Research Tasks |
Ref. |
|
FaDu (pharynx) |
MeWo (granular fibroblasts, derived from human melanoma) |
5:1 |
To study the effect of stromal components on delivery of nanoparticles into the tumors |
[74] |
|
FaDu (pharynx) |
MeWo (granular fibroblasts, derived from human melanoma) |
from 10:1 to 1:2 |
To study penetration, distribution, and antitumor efficacy of photoactive drugs |
[75] |
|
UM-SCC-1 (floor of mouth) |
NHLF (human lung fibroblasts) |
1:1 |
To study the application of high-density lipoprotein nanoparticle as a biocompatible delivery system for a well-established radio-sensitizing miR-34a |
[76] |
|
LK0902 (tongue), LK0917 (gingiva), or LK1108 (hypopharynx) |
CAF (cancer-associated fibroblasts) |
from 2:1 to 3:1 |
To investigate the impact of CAFs on phenotype, proliferation and cisplatin and cetuximab treatment response in HNSCC cells |
[77] |
2.2. Tissue-Engineered Models
2.3. Bioprinted Models
2.4. Organoids
- (1)
-
organoids contain tumor cells at different levels of differentiation, including cancer stem cells;
- (2)
-
organoids consist of several cell types that self-organize in space, reproducing the architectonics of the original tumor tissue;
- (3)
-
self-organization of the organoid occurs in the presence of the ECM;
- (4)
|
|
Perréard, 2023 [112] |
Wang, 2022 [116] |
Driehuis, 2020 [99] |
Kijima, 2019 [117] |
Zhao, 2019 [48] |
Tanaka, 2018 [29] |
|
|
Culture Media |
|||||
|
Basal media |
adDMEM/F12 |
n.d. |
adDMEM/F12 |
adDMEM/F12 |
DMEM/F12 |
StemPro hESC |
|
Penicillin-streptomycin |
100 U/m |
|
100 U/mL |
100 U/mL |
|
|
|
Primocin |
100 μg/mL |
|
|
|
|
|
|
HEPES |
|
|
10 mM |
10 mM |
|
|
|
GlutaMAX |
1× |
|
1× |
1× |
|
|
|
B27 supplement |
1× |
|
1× |
1× |
1× |
|
|
N2 supplement |
|
|
|
1× |
1× |
|
|
N-acetyl-L-cysteine |
1.25 mM |
|
1.25 mM |
0.1 mM |
|
|
|
Nicotinamide |
10 mM |
|
10 mM |
10 nM |
|
|
|
hEGF |
50 ng/mL |
5 ng/mL |
50 ng/mL |
50 ng/mL |
50 ng/mL |
|
|
hFGF-10 |
10 ng/mL |
|
10 ng/mL |
|
|
|
|
hFGF-2 |
5 ng/mL |
5 ng/mL |
5 ng/mL |
|
|
8 ng/mL |
|
A83-01 |
500 nM |
|
500 nM |
500 nM |
500 nM |
|
|
Prostaglandin E2 |
1 μM |
|
1 µM |
|
|
|
|
CHIR-99021 |
0.3 μM |
|
0.3 µM |
|
|
|
|
Forskolin |
1 μM |
|
1 µM |
|
|
|
|
Gastrin |
|
|
|
10 nM |
10 nM |
|
|
Y-27632 |
10 μM |
10 ng/mL |
|
10 μM |
|
|
|
Wnt3A |
|
250 ng/mL |
|
100 ng/mL |
|
|
|
SB202190 |
|
|
|
10 nM |
|
|
|
R-spondin-1 |
|
500 ng/mL |
|
|
|
|
|
Noggin |
|
500 ng/mL |
|
|
|
|
|
R-spondin-1-conditioned media |
10% |
|
|
|
|
|
|
Wnt3a, R-spondin-3, Noggin-conditioned media |
50% |
|
|
|
|
|
|
R-spondin-3-Fc fusion protein conditioned medium |
|
|
4% () |
|
|
|
|
Noggin-Fc fusion protein conditioned medium |
|
|
4% (vol/vol) |
|
|
|
|
Noggin/R-Spondin conditioned media |
|
|
|
2% (vol/vol) |
|
|
|
|
Overall efficacy of organoid generation |
|||||
|
|
assumed around 60% |
62.9% (39/62) |
around 70% |
80% (4/5) |
n.d. |
30.2% (13/43) |
- (1)
-
mass of biomaterial (for tumor samples weighing less than 50 mg, the efficiency of obtaining organoids from it is reduced to 3%);
- (2)
-
time of transportation of tumor tissue to the laboratory (time exceeding 24 h leads to a decrease in efficiency from 60–70% to 22%);
- (3)
-
composition of the culture medium (for example, concentration of Wnt3a, R-spondin-1, EGF, Y27632, Noggin, and FGF2);
- (4)
-
ECM used (Matrigel is preferable to collagen I);
- (5)
-
primary/recurrent tumor status (for nasopharyngeal carcinoma, the efficiency of obtaining organoids is 82% for recurrent tumor and only 47% for primary tumor) [116].
2.5. Tumor Explants and Histocultures
2.6. Microfluidic Devices (Tumor-on-Chip)
|
Object |
In Vitro Culture Duration |
Exposure |
Analysis of System Effluent |
Analysis of the Object |
Ref. |
|
HNSCC biopsies (5–10 mg) |
2 days |
- |
- |
Morphology (H&E staining), cell death (flow cytometry after PI staining), cell viability (MTS proliferation assay) |
[136] |
|
HNSCC biopsies (5–10 mg) |
6 days |
Irradiation (2–40 Gy) |
Cell death (detection of LDH and cytochrome c release) |
Apoptosis (IHC for caspase-cleaved CK18) |
[137] |
|
HNSCC biopsies (5–10 mg) |
2 days |
Irradiation (5–20 Gy) |
Cell death (detection of LDH release) |
Apoptosis (IHC for caspase-cleaved CK18), DNA damage (IHC for phosphorylated-H2AX, TUNEL assay), cell proliferation (IHC for Ki67) |
[138] |
|
HNSCC slices (discs 5×0.35 mm) |
68 h |
Irradiation (5 × 2 Gy), chemotherapy agent (cisplatin) |
Cell death (detection of LDH release) |
Morphology (H&E staining), apoptosis (IHC for caspase-cleaved CK18), DNA damage (IHC for phosphorylated-H2AX), cell proliferation (IHC for Ki67 and BrdU) |
[139] |
|
HNSCC biopsies (5–10 mg) |
9 days |
Chemotherapy agents (cisplatin, 5-flurouracil, docetaxel) |
Cell death (detection of LDH release), cell viability (WST-1 proliferation assay) |
- |
[140] |
|
HNSCC biopsies (5–10 mg) |
7 days |
Chemotherapy agents (cisplatin, 5-flurouracil) |
Cell death (detection of LDH and cytochrome c release), cell viability (WST-1 proliferation assay) |
Morphology (H&E staining) |
[141] |
3. In Vitro Cell Models of HNSCC: Which to Choose?
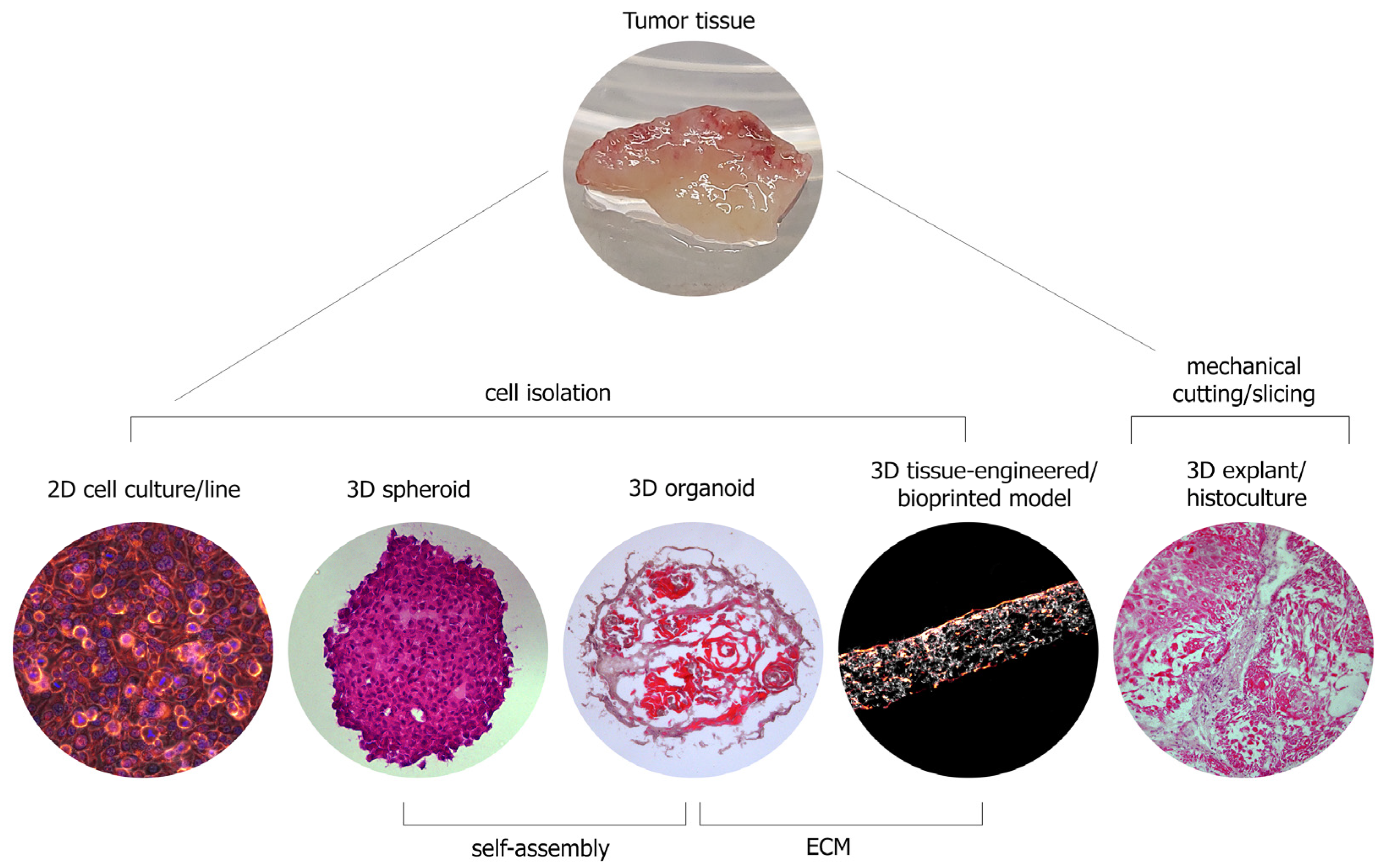
|
|
2D |
3D |
|||||
|
|
Immortalized Cell Lines |
Primary Cell Cultures |
Spheroids and Heterospheroids |
Tissue-Engineered Models |
Bioprinted Models |
Organoids |
Explants and Histocultures |
|
Source |
cell biobanks |
patient-derived tissue |
patient-derived tissue, primary cell cultures, immortalized cell lines |
patient-derived tissue |
|||
|
Heterogeneity of tumor cellular composition |
not preserved |
partially preserved |
depends on the source |
preserved |
|||
|
ЕСМ |
no |
natural and synthetic polymers, decellularized tissue |
bioink based on hydrogels |
basement membrane matrix, collagen |
native |
||
|
Tissue architecture, pathophysiological gradients |
absent |
partially reconstituted |
reconstituted |
preserved |
|||
|
In vitro culture duration |
not limited |
Limited |
|||||
|
Difficulty of obtaining |
low |
medium |
high |
Medium |
|||
|
Major advantages |
availability, stability of properties, many years of experience in use, ability to obtain a 3D model |
availability, ability to obtain a 3D model |
the most available 3D model |
convenience of studying the ECM–cells interaction, possibility of getting a model with given linear dimensions |
obtaining artificial tumor tissue with specified spatial characteristics |
capability to support tumor cells at different levels of differentiation, mimicking the tumor microenvironment |
minimally manipulated tumor tissue |
|
Specific disadvantages |
chromosomal instability, impossibility of use for personalized medicine |
the initial ratio of tumor and tumor-associated cells and their properties may change during cultivation |
prone to fusion to form conglomerates, difficulty in controlling size |
a lot of cells are required for modeling |
a lot of cells are required for modeling, sophisticated equipment is required |
production efficiency about 60–70% |
long-term in vitro cultivation requires supporting matrices or microfluidic devices |
4. In Vitro Cell Models of HNSCC and Oncoviruses
4.1. Human Herpes Viruses (HHVs)
4.2. Human Papillomavirus (HPV)
5. New Trends in In Vitro Modeling of HNSCC
5.1. Models of Vascularization
5.2. New Types of Matrices for Tumor Cell Culturing
6. Three-Dimensional In Vitro Cell Models of HNSCC for Personalized Medicine
7. Conclusions
Funding
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Nissi, L.; Suilamo, S.; Kytö, E.; Vaittinen, S.; Irjala, H.; Minn, H. Recurrence of head and neck squamous cell carcinoma in relation to high-risk treatment volume. Clin. Transl. Radiat. Oncol. 2021, 27, 139–146. [Google Scholar] [CrossRef]
- Albers, A.E.; Grabow, R.; Qian, X.; Jumah, M.D.; Hofmann, V.M.; Krannich, A.; Pecher, G. Efficacy and toxicity of docetaxel combination chemotherapy for advanced squamous cell cancer of the head and neck. Mol. Clin. Oncol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Jin, T.; Qin, W.-F.; Jiang, F.; Jin, Q.-F.; Wei, Q.-C.; Jia, Y.-S.; Sun, X.-N.; Li, W.-F.; Chen, X.-Z. Cisplatin and Fluorouracil Induction Chemotherapy with or without Docetaxel in Locoregionally Advanced Nasopharyngeal Carcinoma. Transl. Oncol. 2019, 12, 633–639. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef] [PubMed
- Boguszewicz, Ł. Predictive Biomarkers for Response and Toxicity of Induction Chemotherapy in Head and Neck Cancers. Front. Oncol. 2022, 12, 900903. [Google Scholar] [CrossRef] [PubMed
- Schanne, D.H.; Koch, A.; Elicin, O.; Giger, R.; Medová, M.; Zimmer, Y.; Aebersold, D.M. Prognostic and Predictive Biomarkers in Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy—A Systematic Review. Biomedicines 2022, 10, 3288. [Google Scholar] [CrossRef] [PubMed
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef] [PubMed
- Chaves, P.; Garrido, M.; Oliver, J.; Pérez-Ruiz, E.; Barragan, I.; Rueda-Domínguez, A. Preclinical models in head and neck squamous cell carcinoma. Br. J. Cancer 2023, 128, 1819–1827. [Google Scholar] [CrossRef] [PubMed
- Seliger, B.; Al-Samadi, A.; Yang, B.; Salo, T.; Wickenhauser, C. In vitro models as tools for screening treatment options of head and neck cancer. Front. Med. 2022, 9, 971726. [Google Scholar] [CrossRef
- Easty, D.M.; Easty, G.C.; Carter, R.L.; Monaghan, P.; Butler, L.J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br. J. Cancer 1981, 43, 772–785. [Google Scholar] [CrossRef
- Burford-Mason, A.P.; Irish, J.C.; MacKay, A.J.; Gullane, P.J.; Bassett, R.; Dardick, I. Squamous cell carcinomas of the head and neck cultured in floating collagen gels: 1. The maintenance of stromal and epithelial elements in vitro without fibroblast overgrowth. Otolaryngol. Neck Surg. 1997, 116, 213–222. [Google Scholar] [CrossRef
- Lepikhova, T.; Karhemo, P.-R.; Louhimo, R.; Yadav, B.; Murumägi, A.; Kulesskiy, E.; Kivento, M.; Sihto, H.; Grénman, R.; Syrjänen, S.M.; et al. Drug-sensitivity screening and genomic characterization of 45 hpV-negative head and neck carcinoma cell lines for novel biomarkers of drug efficacy. Mol. Cancer Ther. 2018, 17, 2060–2071. [Google Scholar] [CrossRef]
- Greaney-Davies, F.S.; Risk, J.M.; Robinson, M.; Liloglou, T.; Shaw, R.J.; Schache, A.G. Essential characterisation of human papillomavirus positive head and neck cancer cell lines. Oral Oncol. 2020, 103, 104613. [Google Scholar] [CrossRef
- Lin, C.J.; Grandis, J.R.; Carey, T.E.; Gollin, S.M.; Whiteside, T.L.; Koch, W.M.; Ferris, R.L.; Lai, S.Y. Head and neck squamous cell carcinoma cell lines: Established models and rationale for selection. Head Neck 2007, 29, 163–188. [Google Scholar] [CrossRef] [PubMed
- Li, H.; Wawrose, J.S.; Gooding, W.E.; Garraway, L.A.; Lui, V.W.Y.; Peyser, N.D.; Grandis, J.R. Genomic analysis of head and neck squamous cell carcinoma cell lines and human tumors: A rational approach to preclinical model selection. Mol. Cancer Res. 2014, 12, 571–582. [Google Scholar] [CrossRef] [PubMed
- Close, D.A.; Wang, A.X.; Kochanek, S.J.; Shun, T.; Eiseman, J.L.; Johnston, P.A. Implementation of the NCI-60 Human Tumor Cell Line Panel to Screen 2260 Cancer Drug Combinations to Generate >3 Million Data Points Used to Populate a Large Matrix of Anti-Neoplastic Agent Combinations (ALMANAC) Database. SLAS Discov. Adv. Sci. Drug Discov. 2019, 24, 242–263. [Google Scholar] [CrossRef] [PubMed
- American Type Culture Collection. Head and Neck Cancer Panel TCP-1012TM. In Medical Radiology; Springer: Cham, Switzerland, 2022; pp. 137–157. [Google Scholar] [CrossRef
- Zhao, M.; Sano, D.; Pickering, C.R.; Jasser, S.A.; Henderson, Y.C.; Clayman, G.L.; Sturgis, E.M.; Ow, T.J.; Lotan, R.; Carey, T.; et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin. Cancer Res. 2011, 17, 7248–7264. [Google Scholar] [CrossRef
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Mseka, T.; Bamburg, J.R.; Cramer, L.P. ADF/cofilin family proteins control formation of oriented actin-filament bundles in the cell body to trigger fibroblast polarization. J. Cell Sci. 2007, 120, 4332–4344. [Google Scholar] [CrossRef]
- Li, C.; Kato, M.; Shiue, L.; Shively, J.E.; Ares, M.; Lin, R.-J. Cell type and culture condition-dependent alternative splicing in human breast cancer cells revealed by splicing-sensitive microarrays. Cancer Res 2006, 66, 1990–1999. [Google Scholar] [CrossRef]
- Gorphe, P. A comprehensive review of Hep-2 cell line in translational research for laryngeal cancer. Am. J. Cancer Res. 2019, 9, 644–649. [Google Scholar]
- Korch, C.T.; Capes-Davis, A. The Extensive and Expensive Impacts of HEp-2 [HeLa], Intestine 407 [HeLa], and Other False Cell Lines in Journal Publications. SLAS Discov. Adv. Sci. Drug Discov. 2021, 26, 1268–1279. [Google Scholar] [CrossRef]
- Tinhofer, I.; Braunholz, D.; Klinghammer, K. Preclinical models of head and neck squamous cell carcinoma for a basic understanding of cancer biology and its translation into efficient therapies. Cancers Head Neck 2020, 5, 9. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Prasad, M.; Kundu, K.; Cohen, L.; Yegodayev, K.M.; Zorea, J.; Joshua, B.-Z.; Lasry, B.; Dimitstein, O.; Bahat-Dinur, A.; et al. Tumor tissue explant culture of patient-derived xenograft as potential prioritization tool for targeted therapy. Front. Oncol. 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-B.; Jung, W.K.; Kim, H.R.; Yu, H.-Y.; Kim, Y.H.; Kim, J. Esculetin has therapeutic potential via the proapoptotic signaling pathway in A253 human submandibular salivary gland tumor cells. Exp. Ther. Med. 2022, 24, 533. [Google Scholar] [CrossRef]
- Colley, H.E.; Hearnden, V.; Jones, A.V.; Weinreb, P.H.; Violette, S.M.; MacNeil, S.; Thornhill, M.H.; Murdoch, C. Development of tissue-engineered models of oral dysplasia and early invasive oral squamous cell carcinoma. Br. J. Cancer 2011, 105, 1582–1592. [Google Scholar] [CrossRef]
- Bernhard, W.; Barreto, K.; El-Sayed, A.; DeCoteau, J.; Geyer, C.R. Imaging Immune Cells Using Fc Domain Probes in Mouse Cancer Xenograft Models. Cancers 2022, 14, 300. [Google Scholar] [CrossRef]
- Bernareggi, D.; Xie, Q.; Prager, B.C.; Yun, J.; Cruz, L.S.; Pham, T.V.; Kim, W.; Lee, X.; Coffey, M.; Zalfa, C.; et al. CHMP2A regulates tumor sensitivity to natural killer cell-mediated cytotoxicity. Nat. Commun. 2022, 13, 1899. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Yang, M.-Y.; Tsai, M.-S.; Chang, G.-H.; Yang, Y.-H.; Tsai, Y.-T.; Wu, C.-Y.; Chang, S.-F. Dihydroisotanshinone i as a treatment option for head and neck squamous cell carcinomas. Int. J. Mol. Sci. 2021, 22, 8881. [Google Scholar] [CrossRef]
- Tevlek, A.; Kecili, S.; Ozcelik, O.S.; Kulah, H.; Tekin, H.C. Spheroid Engineering in Microfluidic Devices. ACS Omega 2023, 8, 3630–3649. [Google Scholar] [CrossRef]
- Huo, K.-G.; D’arcangelo, E.; Tsao, M.-S. Patient-derived cell line, xenograft and organoid models in lung cancer therapy. Transl. Lung Cancer Res. 2020, 9, 2214–2232. [Google Scholar] [CrossRef] [PubMed]
- Moya-Garcia, C.R.; Okuyama, H.; Sadeghi, N.; Li, J.; Tabrizian, M.; Li-Jessen, N.Y.K. In vitro models for head and neck cancer: Current status and future perspective. Front. Oncol. 2022, 12, 960340. [Google Scholar] [CrossRef] [PubMed
- Morrissey, B.; Blyth, K.; Carter, P.; Chelala, C.; Jones, L.; Holen, I.; Speirs, V. The Sharing Experimental Animal Resources, Coordinating Holdings (SEARCH) Framework: Encouraging Reduction, Replacement, and Refinement in Animal Research. PLoS Biol. 2017, 15, e2000719. [Google Scholar] [CrossRef
- Tenschert, E.; Kern, J.; Affolter, A.; Rotter, N.; Lammert, A. Optimisation of Conditions for the Formation of Spheroids of Head and Neck Squamous Cell Carcinoma Cell Lines for Use as Animal Alternatives. Altern. Lab. Anim. 2022, 50, 414–422. [Google Scholar] [CrossRef
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed
- Elliott, N.T.; Yuan, F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 2011, 100, 59–74. [Google Scholar] [CrossRef] [PubMed
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In vitro three-dimensional (3D) models in cancer research: An update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef
- Jung, A.R.; Jung, C.-H.; Noh, J.K.; Lee, Y.C.; Eun, Y.-G. Epithelial-mesenchymal transition gene signature is associated with prognosis and tumor microenvironment in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 3652. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; De Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Thakuri, P.S.; Gupta, M.; Plaster, M.; Tavana, H. Quantitative Size-Based Analysis of Tumor Spheroids and Responses to Therapeutics. ASSAY Drug Dev. Technol. 2019, 17, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-W.; Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef]
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, e1702858. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chang, H. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Mehta, P.; Horst, E.N.; Ward, M.R.; Rowley, K.R.; Mehta, G. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget 2016, 7, 16948–16961. [Google Scholar] [CrossRef]
- Luoto, K.R.; Kumareswaran, R.; Bristow, R.G. Tumor hypoxia as a driving force in genetic instability. Genome Integr. 2013, 4, 5. [Google Scholar] [CrossRef]
- Das, V.; Bruzzese, F.; Konečný, P.; Iannelli, F.; Budillon, A.; Hajdúch, M. Pathophysiologically relevant in vitro tumor models for drug screening. Drug Discov. Today 2015, 20, 848–855. [Google Scholar] [CrossRef]
- Yasui, H.; Kawai, T.; Matsumoto, S.; Saito, K.; Devasahayam, N.; Mitchell, J.B.; Camphausen, K.; Inanami, O.; Krishna, M.C. Quantitative imaging of pO2 in orthotopic murine gliomas: Hypoxia correlates with resistance to radiation. Free. Radic. Res. 2017, 51, 861–871. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Fussenegger, M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004, 22, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A. Experimental anti-tumor therapy in 3-D: Spheroids—Old hat or new challenge? Int. J. Radiat. Biol. 2007, 83, 849–871. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Joshi, R.; Thakuri, P.S.; Tavana, H. Liquid-based three-dimensional tumor models for cancer research and drug discovery. Exp. Biol. Med. 2016, 241, 939–954. [Google Scholar] [CrossRef]
- Santi, M.; Mapanao, A.K.; Cappello, V.; Voliani, V. Production of 3D tumor models of head and neck squamous cell carcinomas for nanotheranostics assessment. ACS Biomater. Sci. Eng. 2020, 6, 4862–4869. [Google Scholar] [CrossRef]
- Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol. Screen. 2006, 11, 922–932. [Google Scholar] [CrossRef]
- Tevis, K.M.; Colson, Y.L.; Grinstaff, M.W. Embedded Spheroids as Models of the Cancer Microenvironment. Adv. Biosyst. 2017, 1, 1700083. [Google Scholar] [CrossRef]
- Hagemann, J.; Jacobi, C.; Gstoettner, S.; Welz, C.; Schwenk-Zieger, S.; Stauber, R.; Strieth, S.; Kuenzel, J.; Baumeister, P.; Becker, S. Therapy testing in a spheroid-based 3D cell culture model for head and neck squamous cell carcinoma. J. Vis. Exp. 2018, 2018, e57012. [Google Scholar] [CrossRef]
- Shao, S.; Scholtz, L.U.; Gendreizig, S.; Martínez-Ruiz, L.; Florido, J.; Escames, G.; Schürmann, M.; Hain, C.; Hose, L.; Mentz, A.; et al. Primary head and neck cancer cell cultures are susceptible to proliferation of Epstein-Barr virus infected lymphocytes. BMC Cancer 2023, 23, 47. [Google Scholar] [CrossRef]
- Hagemann, J.; Jacobi, C.; Hahn, M.; Schmid, V.; Welz, C.; Schwenk-Zieger, S.; Stauber, R.; Baumeister, P.; Becker, S. Spheroid-based 3D cell cultures enable personalized therapy testing and drug discovery in head and neck cancer. Anticancer. Res. 2017, 37, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Vakhshiteh, F.; Bagheri, Z.; Soleimani, M.; Ahvaraki, A.; Pournemat, P.; Alavi, S.E.; Madjd, Z. Heterotypic tumor spheroids: A platform for nanomedicine evaluation. J. Nanobiotechnol. 2023, 21, 249. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Francois, A.; Lamy, L.; Piffoux, M.; Gazeau, F.; Wilhelm, C.; Zorin, V.; Silva, A.K.A.; Bezdetnaya, L. Effect of stroma on the behavior of temoporfin-loaded lipid nanovesicles inside the stroma-rich head and neck carcinoma spheroids. J. Nanobiotechnol. 2021, 19, 3. [Google Scholar] [CrossRef]
- Yakavets, I.; Jenard, S.; Francois, A.; Maklygina, Y.; Loschenov, V.; Lassalle, H.-P.; Dolivet, G.; Bezdetnaya, L. Stroma-rich co-culture multicellular tumor spheroids as a tool for photoactive drugs screening. J. Clin. Med. 2019, 8, 1686. [Google Scholar] [CrossRef]
- Dehghankelishadi, P.; Maritz, M.F.; Badiee, P.; Thierry, B. High density lipoprotein nanoparticle as delivery system for radio-sensitising miRNA: An investigation in 2D/3D head and neck cancer models. Int. J. Pharm. 2022, 617, 121585. [Google Scholar] [CrossRef] [PubMed]
- Magan, M.; Wiechec, E.; Roberg, K. CAFs affect the proliferation and treatment response of head and neck cancer spheroids during co-culturing in a unique in vitro model. Cancer Cell Int. 2020, 20, 599. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Kobayashi, H.; Man, S.; Graham, C.H.; Kapitain, S.J.; Teicher, B.A.; Kerbel, R.S. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc. Natl. Acad. Sci. USA 1993, 90, 3294–3298. [Google Scholar] [CrossRef]
- He, Y.; Deng, P.; Yan, Y.; Zhu, L.; Chen, H.; Li, T.; Li, Y.; Li, J. Matrisome provides a supportive microenvironment for oral squamous cell carcinoma progression. J. Proteom. 2022, 253, 104454. [Google Scholar] [CrossRef]
- Anbazhagan, R.; Sakakura, T.; Gusterson, B.A. The distribution of immuno-reactive tenascin in the epithelial-mesenchymal junctional areas of benign and malignant squamous epithelia. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1990, 59, 59–63. [Google Scholar] [CrossRef]
- Berndt, A.; Hyckel, P.; Kosmehl, H.; Könneker, A. Dreidimensionales In-vitro-Invasionsmodell für orale Plattenepithelkarzinome. Mund-Kiefer-und Gesichtschirurgie 1998, 2, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Corvò, R.; Videtic, G.M.; Paulus, R.; Singh, A.K.; Chang, J.Y.; Parker, W.; Olivier, K.R.; Timmerman, R.D.; Komaki, R.R.; et al. Retinoic acid modulates the radiosensitivity of head-and-neck squamous carcinoma cells grown in collagen gel. Int. J. Radiat. Oncol. 2002, 53, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, G.; Cocchi, C.; De Vita, A.; Liverani, C.; Spadazzi, C.; Calpona, S.; Di Menna, G.; Bassi, M.; Meccariello, G.; De Luca, G.; et al. Three-dimensional collagen-based scaffold model to study the microenvironment and drug-resistance mechanisms of oropharyngeal squamous cell carcinomas. Cancer Biol. Med. 2021, 18, 502–516. [Google Scholar] [CrossRef] [PubMed
- Gu, C.; Zhang, Y.; Chen, D.; Liu, H.; Mi, K. Tunicamycin-induced endoplasmic reticulum stress inhibits chemoresistance of FaDu hypopharyngeal carcinoma cells in 3D collagen I cultures and in vivo. Exp. Cell Res. 2021, 405, 112725. [Google Scholar] [CrossRef] [PubMed
- Young, M.; Rodenhizer, D.; Dean, T.; D’Arcangelo, E.; Xu, B.; Ailles, L.; McGuigan, A.P. A TRACER 3D Co-Culture tumour model for head and neck cancer. Biomaterials 2018, 164, 54–69. [Google Scholar] [CrossRef
- Dean, T.; Li, N.T.; Cadavid, J.L.; Ailles, L.; McGuigan, A.P. A TRACER culture invasion assay to probe the impact of cancer associated fibroblasts on head and neck squamous cell carcinoma cell invasiveness. Biomater. Sci. 2020, 8, 3078–3094. [Google Scholar] [CrossRef
- Ricci, C.; Moroni, L.; Danti, S. Cancer tissue engineering new perspectives in understanding the biology of solid tumours a critical review. OA Tissue Eng. 2013, 1, 1–7. [Google Scholar] [CrossRef
- Wang, J.-Z.; Zhu, Y.-X.; Ma, H.-C.; Chen, S.-N.; Chao, J.-Y.; Ruan, W.-D.; Wang, D.; Du, F.-G.; Meng, Y.-Z. Developing multi-cellular tumor spheroid model (MCTS) in the chitosan/collagen/alginate (CCA) fibrous scaffold for anticancer drug screening. Mater. Sci. Eng. C 2016, 62, 215–225. [Google Scholar] [CrossRef
- Curvello, R.; Kast, V.; Ordóñez-Morán, P.; Mata, A.; Loessner, D. Biomaterial-based platforms for tumour tissue engineering. Nat. Rev. Mater. 2023, 8, 314–330. [Google Scholar] [CrossRef]
- Villasante, A.; Vunjak-Novakovic, G. Tissue-engineered models of human tumors for cancer research. Expert Opin. Drug Discov. 2015, 10, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Schwarz, B.; Forget, A.; Barbero, A.; Martin, I.; Shastri, V.P. Advanced bioink for 3D bioprinting of complex free-standing structures with high stiffness. Bioengineering 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the Cancer Microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98B, 160–170. [Google Scholar] [CrossRef
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef
- Nie, J.; Gao, Q.; Fu, J.; He, Y. Grafting of 3D Bioprinting to In Vitro Drug Screening: A Review. Adv. Healthc. Mater. 2020, 9, e1901773. [Google Scholar] [CrossRef] [PubMed
- Kort-Mascort, J.; Bao, G.; Elkashty, O.; Flores-Torres, S.; Munguia-Lopez, J.G.; Jiang, T.; Ehrlicher, A.J.; Mongeau, L.; Tran, S.D.; Kinsella, J.M. Decellularized Extracellular Matrix Composite Hydrogel Bioinks for the Development of 3D Bioprinted Head and Neck in Vitro Tumor Models. ACS Biomater. Sci. Eng. 2021, 7, 5288–5300. [Google Scholar] [CrossRef] [PubMed
- Kort-Mascort, J.; Shen, M.L.; Martin, E.; Flores-Torres, S.; Pardo, L.A.; Siegel, P.M.; Tran, S.D.; Kinsella, J.M. Bioprinted cancer-stromal in-vitro models in a decellularized ECM-based bioink exhibit progressive remodeling and maturation. Biomed. Mater. 2023, 18, 045022. [Google Scholar] [CrossRef] [PubMed
- Saglam-Metiner, P.; Gulce-Iz, S.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 686, 203–212. [Google Scholar] [CrossRef
- Barbet, V.; Broutier, L. Future Match Making: When Pediatric Oncology Meets Organoid Technology. Front. Cell Dev. Biol. 2021, 9, 674219. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choung, S.; Sun, R.X.; Ung, N.; Hashemi, N.; Fong, E.J.; Lau, R.; Spiller, E.; Gasho, J.; Foo, J.; et al. Comparison of Cell and Organoid-Level Analysis of Patient-Derived 3D Organoids to Evaluate Tumor Cell Growth Dynamics and Drug Response. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 744–754. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Papaccio, F.; Cabeza-Segura, M.; Garcia-Micò, B.; Tarazona, N.; Roda, D.; Castillo, J.; Cervantes, A. Will Organoids Fill the Gap towards Functional Precision Medicine? J. Pers. Med. 2022, 12, 1939. [Google Scholar] [CrossRef]
- Yan, H.H.N.; Siu, H.C.; Law, S.; Ho, S.L.; Yue, S.S.K.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Ma, S.; Lam, K.O.; et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell 2018, 23, 882–897.e811. [Google Scholar] [CrossRef]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kaur, I.; Rawal, P.; Tripathi, D.M.; Vasudevan, A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021, 504, 58–66. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.; London, N.R. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Xia, T.-L.; Tang, H.-C.; Liu, X.; Han, R.; Zou, X.; Zhao, Y.-T.; Chen, M.-Y.; Li, G. Establishment of a patient-derived organoid model and living biobank for nasopharyngeal carcinoma. Ann. Transl. Med. 2022, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Kijima, T.; Nakagawa, H.; Shimonosono, M.; Chandramouleeswaran, P.M.; Hara, T.; Sahu, V.; Kasagi, Y.; Kikuchi, O.; Tanaka, K.; Giroux, V.; et al. Three-Dimensional Organoids Reveal Therapy Resistance of Esophageal and Oropharyngeal Squamous Cell Carcinoma Cells. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 73–91. [Google Scholar] [CrossRef]
- Tuveson, D.A.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef]
- Driehuis, E.; Kolders, S.; Spelier, S.; Lõhmussaar, K.; Willems, S.M.; Devriese, L.A.; de Bree, R.; de Ruiter, E.J.; Korving, J.; Begthel, H.; et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 2019, 9, 852–871. [Google Scholar] [CrossRef]
- Tanaka, N.; Osman, A.A.; Takahashi, Y.; Lindemann, A.; Patel, A.A.; Zhao, M.; Takahashi, H.; Myers, J.N. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol. 2018, 87, 49–57. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, C.-Y.; Chen, W.-M.; Huang, P. Lactate Promotes Cancer Stem-like Property of Oral Sequamous Cell Carcinoma. Curr. Med. Sci. 2019, 39, 403–409. [Google Scholar] [CrossRef]
- Lee, T.W.; Lai, A.; Harms, J.K.; Singleton, D.C.; Dickson, B.D.; Macann, A.M.J.; Hay, M.P.; Jamieson, S.M.F. Patient-derived xenograft and organoid models for precision medicine targeting of the tumour microenvironment in head and neck cancer. Cancers 2020, 12, 3743. [Google Scholar] [CrossRef] [PubMed]
- Bonartsev, A.P.; Lei, B.; Kholina, M.S.; Menshikh, K.A.; Svyatoslavov, D.S.; Samoylova, S.I.; Sinelnikov, M.Y.; Voinova, V.V.; Shaitan, K.V.; Kirpichnikov, M.P.; et al. Models of head and neck squamous cell carcinoma using bioengineering approaches. Crit. Rev. Oncol. 2022, 175, 103724. [Google Scholar] [CrossRef] [PubMed
- Verduin, M.; Hoeben, A.; De Ruysscher, D.; Vooijs, M. Patient-Derived Cancer Organoids as Predictors of Treatment Response. Front. Oncol. 2021, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26.E16. [Google Scholar] [CrossRef] [PubMed
- Park, M.; Kwon, J.; Kong, J.; Moon, S.M.; Cho, S.; Yang, K.Y.; Jang, W.I.; Kim, M.S.; Kim, Y.; Shin, U.S. A patient-derived organoid-based radiosensitivity model for the prediction of radiation responses in patients with rectal cancer. Cancers 2021, 13, 3760. [Google Scholar] [CrossRef
- Smith, R.C.; Tabar, V. Constructing and Deconstructing Cancers using Human Pluripotent Stem Cells and Organoids. Cell Stem Cell 2019, 24, 12–24. [Google Scholar] [CrossRef
- Wu, K.Z.; Adine, C.; Mitriashkin, A.; Aw, B.J.J.; Iyer, N.G.; Fong, E.L.S. Making In Vitro Tumor Models Whole Again. Adv. Healthc. Mater. 2023, 12, e2202279. [Google Scholar] [CrossRef
- Engelmann, L.; Thierauf, J.; Laureano, N.K.; Stark, H.-J.; Prigge, E.-S.; Horn, D.; Freier, K.; Grabe, N.; Rong, C.; Federspil, P.; et al. Organotypic co-cultures as a novel 3d model for head and neck squamous cell carcinoma. Cancers 2020, 12, 2330. [Google Scholar] [CrossRef
- Dohmen, A.J.; Sanders, J.; Canisius, S.; Jordanova, E.S.; Aalbersberg, E.A.; Brekel, M.W.v.D.; Neefjes, J.; Zuur, C.L. Sponge-supported cultures of primary head and neck tumors for an optimized preclinical model. Oncotarget 2018, 9, 25034–25047. [Google Scholar] [CrossRef
- Lee, J.; You, J.H.; Shin, D.; Roh, J.-L. Ex vivo culture of head and neck cancer explants in cell sheet for testing chemotherapeutic sensitivity. J. Cancer Res. Clin. Oncol. 2020, 146, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Demers, I.; Donkers, J.; Kremer, B.; Speel, E.J. Ex Vivo Culture Models to Indicate Therapy Response in Head and Neck Squamous Cell Carcinoma. Cells 2020, 9, 2527. [Google Scholar] [CrossRef] [PubMed
- Peria, M.; Donnadieu, J.; Racz, C.; Ikoli, J.; Galmiche, A.; Chauffert, B.; Page, C. Evaluation of individual sensitivity of head and neck squamous cell carcinoma to cetuximab by short-term culture of tumor slices. Head Neck 2016, 38, E911–E915. [Google Scholar] [CrossRef] [PubMed
- Gerlach, M.M.; Merz, F.; Wichmann, G.; Kubick, C.; Wittekind, C.; Lordick, F.; Dietz, A.; Bechmann, I. Slice cultures from head and neck squamous cell carcinoma: A novel test system for drug susceptibility and mechanisms of resistance. Br. J. Cancer 2014, 110, 479–488. [Google Scholar] [CrossRef
- Bower, R.; Green, V.L.; Kuvshinova, E.; Kuvshinov, D.; Karsai, L.; Crank, S.T.; Stafford, N.D.; Greenman, J. Maintenance of head and neck tumor on-chip: Gateway to personalized treatment? Futur. Sci. OA 2017, 3, FSO174. [Google Scholar] [CrossRef
- Carr, S.D.; Green, V.L.; Stafford, N.D.; Greenman, J. Analysis of radiation-induced cell death in head and neck squamous cell carcinoma and rat liver maintained in microfluidic devices. Otolaryngol. Neck Surg. 2014, 150, 73–80. [Google Scholar] [CrossRef
- Cheah, R.; Srivastava, R.; Stafford, N.D.; Beavis, A.W.; Green, V.; Greenman, J. Measuring the response of human head and neck squamous cell carcinoma to irradiation in a microfluidic model allowing customized therapy. Int. J. Oncol. 2017, 51, 1227–1238. [Google Scholar] [CrossRef]
- Kennedy, R.; Kuvshinov, D.; Sdrolia, A.; Kuvshinova, E.; Hilton, K.; Crank, S.; Beavis, A.W.; Green, V.; Greenman, J. A patient tumour-on-a-chip system for personalised investigation of radiotherapy based treatment regimens. Sci. Rep. 2019, 9, 6327. [Google Scholar] [CrossRef]
- Sylvester, D.; Hattersley, S.M.; Stafford, N.D.; Haswell, S.J.; Greenman, J. Development of Microfluidic-based Analytical Methodology for Studying the Effects of Chemotherapy Agents on Cancer Tissue. Curr. Anal. Chem. 2012, 9, 2–8. [Google Scholar] [CrossRef]
- Hattersley, S.M.; Sylvester, D.C.; Dyer, C.E.; Stafford, N.D.; Haswell, S.J.; Greenman, J. A microfluidic system for testing the responses of head and neck squamous cell carcinoma tissue biopsies to treatment with chemotherapy drugs. Ann. Biomed. Eng. 2012, 40, 1277–1288. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Heyder, P.; Schroeder, J.; Knuechel, R. A heterologous 3-D coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation. Exp. Cell Res. 2001, 266, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef] [PubMed]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef]
- Kochanek, S.J.; Close, D.A.; Camarco, D.P.; Johnston, P.A. Maximizing the Value of Cancer Drug Screening in Multicellular Tumor Spheroid Cultures: A Case Study in Five Head and Neck Squamous Cell Carcinoma Cell Lines. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 329–349. [Google Scholar] [CrossRef]
- Storch, K.; Eke, I.; Borgmann, K.; Krause, M.; Richter, C.; Becker, K.; Schröck, E.; Cordes, N. Three-dimensional cell growth confers radioresistance by chromatin density modification. Cancer Res 2010, 70, 3925–3934. [Google Scholar] [CrossRef]
- Eke, I.; Schneider, L.; Förster, C.; Zips, D.; Kunz-Schughart, L.A.; Cordes, N. EGFR/JIP-4/JNK2 signaling attenuates cetuximab-mediated radiosensitization of squamous cell carcinoma cells. Cancer Res. 2013, 73, 297–306. [Google Scholar] [CrossRef]
- Eke, I.; Leonhardt, F.; Storch, K.; Hehlgans, S.; Cordes, N. The small molecule inhibitor QLT0267 radiosensitizes squamous cell carcinoma cells of the head and neck. PLoS ONE 2009, 4, e6434. [Google Scholar] [CrossRef]
- Affolter, A.; Lammert, A.; Kern, J.; Scherl, C.; Rotter, N. Precision Medicine Gains Momentum: Novel 3D Models and Stem Cell-Based Approaches in Head and Neck Cancer. Front. Cell Dev. Biol. 2021, 9, 666515. [Google Scholar] [CrossRef]
- Schmidt, M.; Scholz, C.-J.; Polednik, C.; Roller, J. Spheroid-based 3-dimensional culture models: Gene expression and functionality in head and neck cancer. Oncol. Rep. 2016, 35, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Close, D.A.; Camarco, D.P.; Johnston, P.A. High-Content Screening Comparison of Cancer Drug Accumulation and Distribution in Two-Dimensional and Three-Dimensional Culture Models of Head and Neck Cancer. ASSAY Drug Dev. Technol. 2018, 16, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Mahmutović, L.; Bilajac, E.; Hromić-Jahjefendić, A. Meet the insidious players: Review of viral infections in head and neck cancer etiology with an update on clinical trials. Microorganisms 2021, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein–Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160270. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Świętek, A.; Hudy, D.; Gołąbek, K.; Gaździcka, J.; Miśkiewicz-Orczyk, K.; Ścierski, W.; Strzelczyk, J.; Misiołek, M. Low Prevalence of HSV-1 and Helicobacter pylori in HNSCC and Chronic Tonsillitis Patients Compared to Healthy Individuals. Diagnostics 2023, 13, 1798. [Google Scholar] [CrossRef]
- Forslund, O.; Sugiyama, N.; Wu, C.; Ravi, N.; Jin, Y.; Swoboda, S.; Andersson, F.; Bzhalava, D.; Hultin, E.; Paulsson, K.; et al. A novel human in vitro papillomavirus type 16 positive tonsil cancer cell line with high sensitivity to radiation and cisplatin. BMC Cancer 2019, 19, 265. [Google Scholar] [CrossRef]
- Wegge, M.; Dok, R.; Dubois, L.J.; Nuyts, S. Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 3763. [Google Scholar] [CrossRef]
- Vitti, E.T.; Kacperek, A.; Parsons, J.L. Targeting DNA double-strand break repair enhances radiosensitivity of HPV-positive and HPV-negative head and neck squamous cell carcinoma to photons and protons. Cancers 2020, 12, 1490. [Google Scholar] [CrossRef]
- Millen, R.; De Kort, W.W.; Koomen, M.; van Son, G.J.; Gobits, R.; de Vries, B.P.; Begthel, H.; Zandvliet, M.; Doornaert, P.; Raaijmakers, C.P.; et al. Patient-derived head and neck cancer organoids allow treatment stratification and serve as a tool for biomarker validation and identification. Med 2023, 4, 290–310.e12. [Google Scholar] [CrossRef]
- Facompre, N.D.; Rajagopalan, P.; Sahu, V.; Pearson, A.T.; Montone, K.T.; James, C.D.; Gleber-Netto, F.O.; Weinstein, G.S.; Jalaly, J.; Lin, A.; et al. Identifying predictors of HPV-related head and neck squamous cell carcinoma progression and survival through patient-derived models. Int. J. Cancer 2020, 147, 3236–3249. [Google Scholar] [CrossRef]
- Almela, T.; Tayebi, L.; Moharamzadeh, K. 3D bioprinting for in vitro models of oral cancer: Toward development and validation. Bioprinting 2021, 22, e00132. [Google Scholar] [CrossRef]
- Matuszczak, S.; Szczepanik, K.; Grządziel, A.; Drzyzga, A.; Cichoń, T.; Czapla, J.; Pilny, E.; Smolarczyk, R. The Effect of Radiotherapy on Cell Survival and Inflammatory Cytokine and Chemokine Secretion in a Co-Culture Model of Head and Neck Squamous Cell Carcinoma and Normal Cells. Biomedicines 2023, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Yerneni, S.S.; Razzo, B.M.; Whiteside, T.L. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Mol. Cancer Res. 2018, 16, 1798–1808. [Google Scholar] [CrossRef] [PubMed
- Choi, S.-Y.; Kang, S.H.; Oh, S.Y.; Lee, K.Y.; Lee, H.-J.; Gum, S.; Kwon, T.-G.; Kim, J.-W.; Lee, S.-T.; Hong, Y.J.; et al. Differential angiogenic potential of 3-dimension spheroid of hnscc cells in mouse xenograft. Int. J. Mol. Sci. 2021, 22, 8245. [Google Scholar] [CrossRef] [PubMed
- Bessho, T.; Takagi, T.; Igawa, K.; Sato, K. Gelatin-based cell culture device for construction and X-ray irradiation of a three-dimensional oral cancer model. Anal. Sci. 2023, 39, 771–778. [Google Scholar] [CrossRef
- Yang, J.; Wang, W.; Xia, H.; Yu, Z.; Li, H.; Ren, J.; Chen, G.; Wang, B.; Jia, J.; Zhang, W.; et al. Lymphotoxin-α promotes tumor angiogenesis in HNSCC by modulating glycolysis in a PFKFB3-dependent manner. Int. J. Cancer 2019, 145, 1358–1370. [Google Scholar] [CrossRef
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising applications of tumor spheroids and organoids for personalized medicine. Cancers 2020, 12, 2727. [Google Scholar] [CrossRef
- Ehsan, S.M.; Welch-Reardon, K.M.; Waterman, M.L.; Hughes, C.C.W.; George, S.C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. 2014, 6, 603–610. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Verbridge, S.S.; Vlachos, P.P.; Rylander, M.N. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adhes. Migr. 2014, 8, 517–524. [Google Scholar] [CrossRef]
- Cauli, E.; Polidoro, M.A.; Marzorati, S.; Bernardi, C.; Rasponi, M.; Lleo, A. Cancer-on-chip: A 3D model for the study of the tumor microenvironment. J. Biol. Eng. 2023, 17, 53. [Google Scholar] [CrossRef]
- Clarke, R. Introduction: Cancer Systems and Integrative Biology; Humana: New York, NY, USA, 2023; Volume 2660. [Google Scholar] [CrossRef
- Tuomainen, K.; Al-Samadi, A.; Potdar, S.; Turunen, L.; Turunen, M.; Karhemo, P.-R.; Bergman, P.; Risteli, M.; Åström, P.; Tiikkaja, R.; et al. Human tumor–derived matrix improves the predictability of head and neck cancer drug testing. Cancers 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed
- Naakka, E.; Wahbi, W.; Tiikkaja, R.; Juurikka, K.; Sandvik, T.; Koivunen, P.; Autio, T.; Tikanto, J.; Väisänen, J.; Tuominen, H.; et al. Novel human lymph node-derived matrix supports the adhesion of metastatic oral carcinoma cells. BMC Cancer 2023, 23, 750. [Google Scholar] [CrossRef] [PubMed
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed
- Pillai, S.; Kwan, J.C.; Yaziji, F.; Yu, H.; Tran, S.D. Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers. Cancers 2023, 15, 3894. [Google Scholar] [CrossRef
- Sawant, S.; Dongre, H.; Singh, A.K.; Joshi, S.; Costea, D.E.; Mahadik, S.; Ahire, C.; Makani, V.; Dange, P.; Sharma, S.; et al. Establishment of 3D co-culture models from different stages of human tongue tumorigenesis: Utility in understanding neoplastic progression. PLoS ONE 2016, 11, e0160615. [Google Scholar] [CrossRef]
- Establishment of Squamous Cell Organoids of the Head and Neck to Assess Their Response to Innovative Therapies (ORGAVADS); ClinicalTrials.gov Identifier: NCT04261192. Available online: https://clinicaltrials.gov/study/NCT04261192 (accessed on 1 October 2023).
- Perréard, M.; Florent, R.; Divoux, J.; Grellard, J.-M.; Lequesne, J.; Briand, M.; Clarisse, B.; Rousseau, N.; Lebreton, E.; Dubois, B.; et al. ORGAVADS: Establishment of tumor organoids from head and neck squamous cell carcinoma to assess their response to innovative therapies. BMC Cancer 2023, 23, 223. [Google Scholar] [CrossRef]
- Selecting Chemotherapy with High-Throughput Drug Screen Assay Using Patient Derived Organoids in Patients with Refractory Solid Tumours (SCORE); ClinicalTrials.gov Identifier: NCT04279509. Available online: https://clinicaltrials.gov/study/NCT04279509 (accessed on 1 October 2023).
- SOTO: Treatment Sensitivity of Organoids to Predict Treatment Outcome; ClinicalTrials.gov Identifier: NCT05400239. Available online: https://classic.clinicaltrials.gov/ct2/history/NCT05400239?V_2=View (accessed on 1 October 2023).
