- genetic conditions
1. Presentation
Clinical features of IPO can include abdominal pain, nausea, abdominal distension, vomiting, dysphagia, and constipation. Symptoms depend on the portion of the gastrointestinal tract involved[2] and the duration of symptoms. Symptoms may occur intermittently and over a prolonged period of time. It is not unusual for patients to present several times owing to the nonspecific nature of the symptoms.[4] Conditions and onset will vary if the disease is primary vs secondary and the underlying disease (if a secondary manifestation) and its management.
Symptoms indicative of advanced disease and possible intestinal failure include diarrhea, loss of appetite, sepsis, bloating, fatigue, signs of low volume status, and malabsorption including nutritional deficiencies and foul-smelling stools.[13][14]
2. Causes
In primary CIPO (the majority of chronic cases) the condition results from disruption of the intestine's ability to move food. These can be broadly classified as myopathic (affecting the smooth muscle), mesenchymopathic (affecting the interstitial cells of Cajal), or neuropathic (of the nervous system) of the gastrointestinal tract.[15]
In some cases there appears to be a genetic association.[16] One form has been associated with DXYS154.[17]
Secondary chronic intestinal pseudo-obstruction can occur as a consequence of a number of other conditions including:
- Hirschsprung's disease[18] - the absence of colonic nerve cells
- Chagas' disease - a chronic parasitic infection of the colon leading to loss of nerve endings
- Kawasaki disease[19][20] - a rare presentation for this particular autoimmune disorder of the vasculature
- Parkinson's disease[21] - related to the neurodegeneration of gastrointestinal tract
- Autoimmune conditions - conditions including systemic lupus erythematosus and scleroderma lead to collagen vascular deposition[22] and gastrointestinal motility disruption
- Mitochondrial disease[23] - IPO is a known presentation for mitochondrial disease
- Endocrine disorders[4]
- Certain medications.[15]
The term may be used synonymously with enteric neuropathy if a neurological cause is suspected.
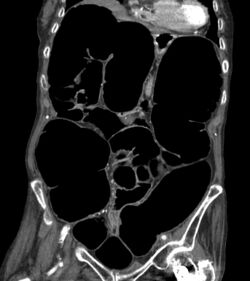
3. Diagnosis
The symptoms of IPO are nonspecific. It is not unusual for patients to present repeatedly and to undergo numerous tests.[4] Mechanical causes of intestinal obstruction must be excluded to reach a diagnosis of pseudo-obstruction. Attempts must also be made to determine whether the IPO is the result of a primary or secondary condition.[15] A diagnostic work-up may include:[14]
- Gastric motility studies
- Imaging studies:
- X-rays - may show intestinal air fluid levels (seen with true mechanical intestinal obstruction)
- CT scans
- Barium enema
- Blood tests
- Upper and lower endoscopies
- Manometry - used to measure pressure of esophagus and stomach
4. Treatment
Treatment for IPO (acute or chronic) is aimed at removing the disease process and/or managing the complications present. Focus is placed on management of pain, gastrointestinal symptoms, nutritional deficiencies, fluid status, infection control, and improving quality of life. When CIPO is secondary to another disease, treatment is addressed towards the underlying condition. Surgery is sometimes required in severe cases of CIPO.
Medical treatment
Prucalopride,[24][25] pyridostigmine,[11] metoclopramide, cisapride, erythromycin[9] and octreotide[9][26][27] are medications that aim to enhance intestinal motility.
Intestinal stasis, which may lead to bacterial overgrowth and subsequently, diarrhea or malabsorption, is treated with antibiotics.
Nutritional deficiencies are treated by encouraging patients to avoid foods that increase distention and are difficult to digest (e.g. those high in fat and fibre), consuming small frequent meals (5–6 per day), focusing on liquids and soft food. Reducing intake of poorly absorbed sugar alcohols may be of benefit. Referral to an accredited dietitian is recommended. If dietary changes are unsuccessful in meeting nutritional requirements and energy needs, enteral nutrition is used. Many patients eventually require parenteral nutrition.[15]
Total parenteral nutrition (TPN) is a form of long-term nutritional treatment reserved for patients that have severe pseudo-obstruction. TPN dependent patients require frequent checkups to monitor catheter function, check liver enzyme levels, and evaluate for signs of blood infections. TPN format is typically changed depending on loss/gain of weight and bloodwork results, and is specially formulated to meet each individual patient's needs.[28]
Procedures
Intestinal decompression by tube placement in a small stoma can also be used to reduce distension and pressure within the gut. The stoma may be a gastrostomy, jejunostomy, ileostomy or cecostomy. These may be used for feed (e.g. gastrostomy and jejunostomy) or to flush the intestines.
Colostomy or ileostomy can bypass affected parts if they are distal to (come after) the stoma. For instance, if only the colon is affected, an ileostomy may be helpful. Either of these ostomies are typically placed at or a few centimeters below the patient's navel per doctor recommendation based on the affected area of the intestines as well as concerns for patient comfort and future physical growth for children.[28]
The total removal of the colon, called a colectomy or resection of affected parts of the colon may be needed if part of the gut dies (for instance toxic megacolon), or if there is a localized area of dysmotility.
Gastric and colonic pacemakers have been tried. These are strips placed along the colon or stomach which create an electric discharge intended to cause the muscle to contract in a controlled manner.
A potential solution, albeit radical, is intestinal transplantation. This is only appropriate in the case of intestinal failure. These procedures are most frequently described in pediatric cases of CIPO.[29][30] One operation involving multi-organ transplant of the pancreas, stomach, duodenum, small intestine, and liver, and was performed by Doctor Kareem Abu-Elmagd on Gretchen Miller.[31]
Potential Treatments
Further research is necessary into other treatments which may alleviate symptoms. These include stem-cell transplantation[9][32][33] and fecal microbiota transplantation.[9] Cannabis[34][35] has not been studied with regards to CIPO. Any claims to its efficacy for use in CIPO are speculative.
5. Related Disorders
- Ogilvie syndrome: acute pseudoobstruction of the colon in severely ill debilitated patients.
- Hirschsprung's disease: enlargement of the colon due to lack of development of autonomic ganglia.
- Intestinal neuronal dysplasia: a disease of motor neurons leading to the bowels.
- Bowel obstruction: mechanical or functional obstruction of the bowel, most commonly due to adhesions, hernias or neoplasms.
- Enteric neuropathy: alternative name sometimes used for diagnosis in UK
This entry is adapted from the peer-reviewed paper https://medlineplus.gov/genetics/condition/intestinal-pseudo-obstruction
References
- Gargiulo A, Auricchio R, Barone MV, Cotugno G, Reardon W, Milla PJ, BallabioA, Ciccodicola A, Auricchio A. Filamin A is mutated in X-linked chronicidiopathic intestinal pseudo-obstruction with central nervous system involvement.Am J Hum Genet. 2007 Apr;80(4):751-8.
- Gauthier J, Ouled Amar Bencheikh B, Hamdan FF, Harrison SM, Baker LA, Couture F, Thiffault I, Ouazzani R, Samuels ME, Mitchell GA, Rouleau GA, Michaud JL,Soucy JF. A homozygous loss-of-function variant in MYH11 in a case withmegacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Hum Genet. 2015 Sep;23(9):1266-8. doi: 10.1038/ejhg.2014.256.
- Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, Akdemir ZHC, Doukas M, Stoop HJ, de Graaf BM, Brouwer RWW, van Ijcken WFJ, Oury JF, RosenblattJ, Burns AJ, Tibboel D, Hofstra RMW, Alves MM. Loss-of-Function Variants in MYLK Cause Recessive Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. Am JHum Genet. 2017 Jul 6;101(1):123-129. doi: 10.1016/j.ajhg.2017.05.011.
- Halim D, Wilson MP, Oliver D, Brosens E, Verheij JB, Han Y, Nanda V, Lyu Q,Doukas M, Stoop H, Brouwer RW, van IJcken WF, Slivano OJ, Burns AJ, Christie CK, de Mesy Bentley KL, Brooks AS, Tibboel D, Xu S, Jin ZG, Djuwantono T, Yan W,Alves MM, Hofstra RM, Miano JM. Loss of LMOD1 impairs smooth musclecytocontractility and causes megacystis microcolon intestinal hypoperistalsissyndrome in humans and mice. Proc Natl Acad Sci U S A. 2017 Mar28;114(13):E2739-E2747. doi: 10.1073/pnas.1620507114.
- Iida H, Ohkubo H, Inamori M, Nakajima A, Sato H. Epidemiology and clinicalexperience of chronic intestinal pseudo-obstruction in Japan: a nationwideepidemiologic survey. J Epidemiol. 2013;23(4):288-94.
- Kapoor S. Kawasaki's disease: an often overlooked cause of intestinalpseudo-obstruction in children. Virchows Arch. 2015 Nov;467(5):619-20. doi:10.1007/s00428-015-1844-2.
- Kapur RP, Robertson SP, Hannibal MC, Finn LS, Morgan T, van Kogelenberg M,Loren DJ. Diffuse abnormal layering of small intestinal smooth muscle is present in patients with FLNA mutations and x-linked intestinal pseudo-obstruction. Am J Surg Pathol. 2010 Oct;34(10):1528-43. doi: 10.1097/PAS.0b013e3181f0ae47.
- Klar J, Raykova D, Gustafson E, Tóthová I, Ameur A, Wanders A, Dahl N.Phenotypic expansion of visceral myopathy associated with ACTG2 tandem basesubstitution. Eur J Hum Genet. 2015 Dec;23(12):1679-83. doi:10.1038/ejhg.2015.49.
- Lauro A, De Giorgio R, Pinna AD. Advancement in the clinical management ofintestinal pseudo-obstruction. Expert Rev Gastroenterol Hepatol. 2015Feb;9(2):197-208. doi: 10.1586/17474124.2014.940317.
- Lehtonen HJ, Sipponen T, Tojkander S, Karikoski R, Järvinen H, Laing NG,Lappalainen P, Aaltonen LA, Tuupanen S. Segregation of a missense variant inenteric smooth muscle actin γ-2 with autosomal dominant familial visceralmyopathy. Gastroenterology. 2012 Dec;143(6):1482-1491.e3. doi:10.1053/j.gastro.2012.08.045.
- Matera I, Rusmini M, Guo Y, Lerone M, Li J, Zhang J, Di Duca M, Nozza P,Mosconi M, Pini Prato A, Martucciello G, Barabino A, Morandi F, De Giorgio R,Stanghellini V, Ravazzolo R, Devoto M, Hakonarson H, Ceccherini I. Variants ofthe ACTG2 gene correlate with degree of severity and presence of megacystis inchronic intestinal pseudo-obstruction. Eur J Hum Genet. 2016 Aug;24(8):1211-5.doi: 10.1038/ejhg.2015.275.
- Milunsky A, Baldwin C, Zhang X, Primack D, Curnow A, Milunsky J. Diagnosis of Chronic Intestinal Pseudo-obstruction and Megacystis by Sequencing the ACTG2Gene. J Pediatr Gastroenterol Nutr. 2017 Oct;65(4):384-387. doi:10.1097/MPG.0000000000001608.
- Wangler MF, Gonzaga-Jauregui C, Gambin T, Penney S, Moss T, Chopra A, ProbstFJ, Xia F, Yang Y, Werlin S, Eglite I, Kornejeva L, Bacino CA, Baldridge D, Neul J, Lehman EL, Larson A, Beuten J, Muzny DM, Jhangiani S; Baylor-Hopkins Centerfor Mendelian Genomics, Gibbs RA, Lupski JR, Beaudet A. Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underliemegacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet. 2014 Mar27;10(3):e1004258. doi: 10.1371/journal.pgen.1004258.