Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Airway mucus is a complex viscoelastic gel mainly composed of water, glycoproteins, lipids, enzymes, minerals, etc. Among them, glycoprotein is the main factor determining mucus-gel-like rheology. Airway mucus forms a protective barrier by secreting mucin, which represents the absorption barrier, especially for more lipophilic drugs. It rapidly clears the drug from the airways through physiological mucus clearance mechanisms, so the drug does not remain in the lungs or reach the airway epithelial tissue for a long time.
- airway mucus
- nanoparticles
1. Introduction
The mucus layer plays a vital role in human health, as it is the front line of the body's defense system [1], capable of selectively penetrating foreign bodies and pathogens, thereby protecting the normal functioning of the organism [2]. Lung mucus consists of two layers: the fluid layer on the airway surface and the layer around the eyelashes. The former consists of gel-forming mucus and is responsible for adsorbing and encapsulating inhalation particles. The latter is where surface cells beat and relax, efficiently transporting the mucus layer to the outside of the lungs [3][4]. The composition and thickness of the mucus layer is not constant; It is a dynamic system whose composition and thickness are caused by the continuous secretion and clearance of mucus [5].
In addition to water, the main component of mucus is mucin, which can be divided into two subtypes according to its glycosylation variability: secreted mucin and membrane-bound mucin. Membrane-binding proteins bind mainly to the surface of the mucosal epithelium, while the disulfide bonds between them link the secreted mucins to form a continuous gel state [6]. Given the importance of lung mucus to the bioavailability of pulmonary drugs, studying the important components and tissues of lung mucus [5] is critical to understanding its barrier function. Drugs delivered directly to the airways or inhalation therapy [7] are commonly used to treat lung disease. Among them, inhalation therapy makes it easier to deliver therapeutic drugs to the pulmonary mucosa, so it is possible to significantly reduce the dose of the drug and further reduce its side effects. However, due to the inherent clearance mechanism of the pulmonary mucosa, the drug is less bioavailable in the lungs, and most of the drug is cleared in the pulmonary mucosa [8][9].
Over the past few decades, convincing data have confirmed that nanodelivery systems can be promising carriers for delivering drugs through the mucus layer, including polymer nanoparticles, liposomes, polymer micelles, and nanoparticles [10][11][12][13][14][15]. After inhalation delivery to the airway, these nanoparticles can penetrate or remain in the airway mucus layer by osmosis-mediated, adhesion-mediated, and biomimetic-mediated, respectively [16][17][18]. The benefits of utilizing nanocarriers are increased bioavailability of the drug and reduced unwanted toxicity due to its surface modification, suitable nanometer size, and blood stability [19][20].
2. Pathophysiology of Airway Mucus
According to statistics, a typical adult breaths about 16-20 times per minute in a calm state. The amount of gas inhaled or exhaled is approximately 500 mL, or tidal volume [21]. As a result, the surface of the human airways constantly interacts with the external environment, including inhaled particles and pathogens. The mucociliary clearance mechanism is an important defense mechanism for maintaining a normal physiological state of the human airway [22]. The mucocilia removal system avoids the retention of pathogenic bacteria by constantly renewing the mucus blanket while removing pathogenic microorganisms and inhaled particles. The mucosal cilia clearance system [23] has three main components: the surface fluid layer in contact with the airway lumen, the periciliary fluid layer that supports cilia beating, and the respiratory epithelium composed of secretory cells [24] (Figure 1).
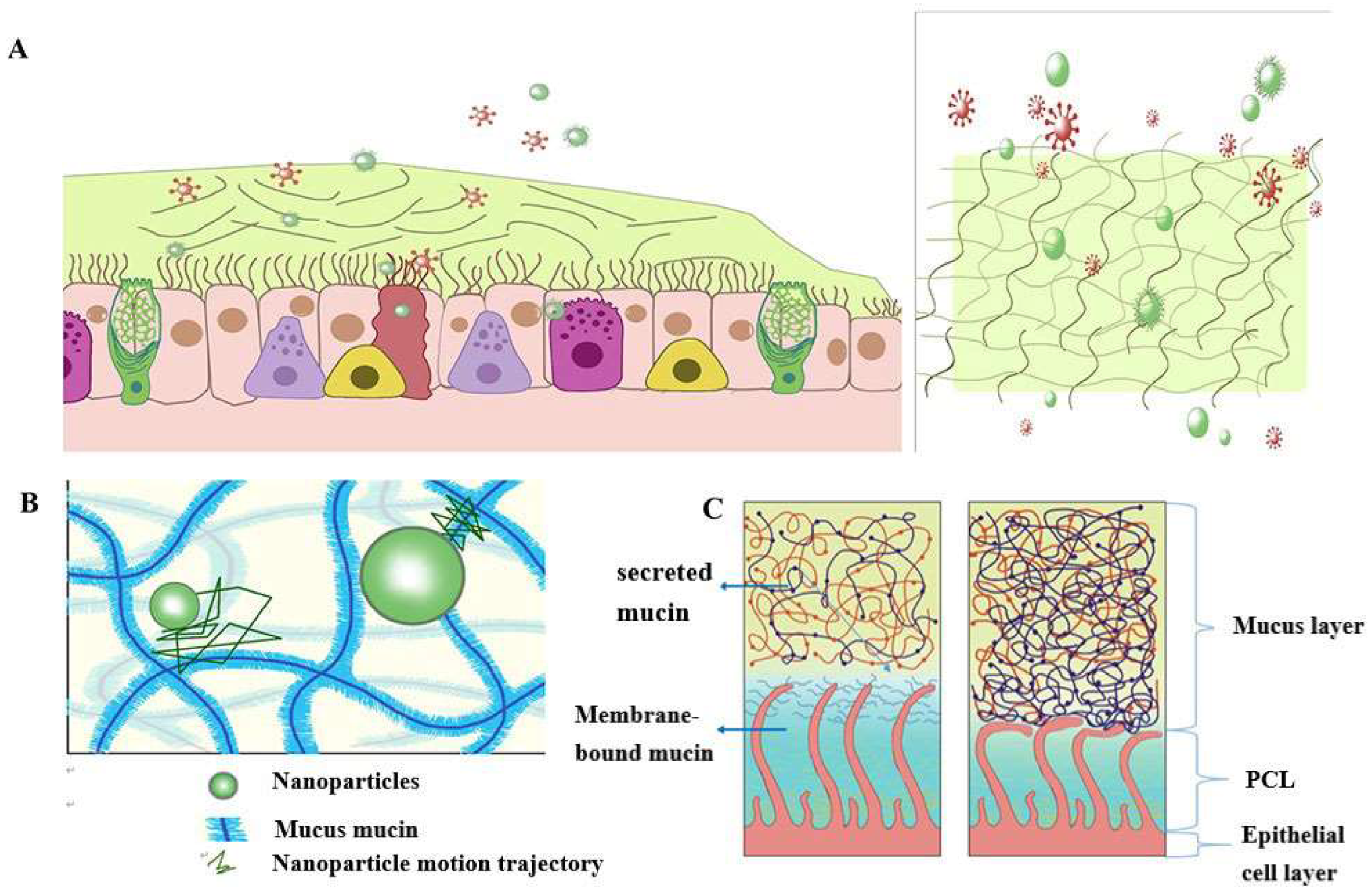
Figure 1Schematic diagram of the structure and function of the mucus barrier: (A) The mucosal cilia removal system has three main components: the surface fluid layer in contact with the airway lumen, the periciliary fluid layer that supports cilia beating, and the respiratory epithelium composed of secretory cells. Constant renewal of the mucus blanket avoids the retention of pathogenic bacteria, while removing pathogenic microorganisms (red spheres) and inhaled particulate matter (green spheres); (B) Schematic diagram of the nanoparticle structure through the mucin network. Small molecules can cross the mucus barrier by diffusion freely, but most large molecules do not easily cross the mucus barrier; (C) Schematic diagram of PCL collapse under normal conditions due to mucus and PCL layers and airway dehydration.
The mucus layer is one of the key components of the mucocilia removal system, which acts as both a physical and a chemical barrier. The mucus layer consists of hundreds of substances and contains 98% water and 2% solids [25]. The main macromolecule of this 2% solid substance is mucin, a macromolecule formed from a family of glycoproteins that are highly glycosylated [26][27][28]. The main secreted mucins in the airways are MUC5B and MUC5AC [29][30], which have characteristic domains formed by repeated tandem associations of abundant proline, serine, serine, and threonine. The repeat sequence undergoes O-glycosylation to harden the mucin backbone and increase the stiffness of the mucin chain, thus maintaining the gel morphology of the mucus. Mucin itself can bind to therapeutic drugs in a non-specific way. Pavan G. Bhat and colleagues studied the permeability of porcine gastric mucus to five substances: isoniazid [31], pentamidine [32], rifampicin [33], p-aminosalicylic acid [34], and pyrazinamide [35], all of which can be delivered to lung targets using inhalation therapy. The permeability of all two agents was significantly reduced in the presence of mucus compared with the permeability of the blank buffer [2]. The above results show that all compounds bind specifically to mucin molecules before passing through the mucus layer, resulting in reduced penetration through the mucus layer. The pore size of the mucus layer also plays a crucial role in the penetration of the drug. Anionic and nonionic surfactants have a more significant effect on the mucus permeability of nanoparticles and their mucus barrier modulation ability, which also depends on the type of surfactant. Sodium lauryl sulfate (SDS) increased the composite viscosity and viscoelasticity of mucus, but poloxamer showed a downward trend. Tween 80 largely retains its original mucus rheological and morphological properties and may be a promising candidate for promoting the penetration of nanoparticles into the mucus barrier with good safety. Studies have shown that some small molecules can cross the mucus barrier through free diffusion, but most large molecules do not easily cross the mucus barrier [27]. Therefore, the permeability of the mucus layer may be limited by its pore size can be infered [36].
The periciliated liquid layer (PCL) is ideal for cilia flow and is approximately 5-10 μm thick, [4] corresponding to the length of the cilia. If the layer is too thick, cilia cannot reach the upper mucus layer and therefore cannot perform their clearing defense function. At the same time, if this layer is too thin, the upper mucus layer adheres to the cilia and blocks their movement [23]. Hydration of airway surface fluids is critical to achieving mean mucus clearance [25]; In the normal airway [37], water is distributed between the mucus layer and the periciliary fluid layer, and the layer with low osmotic pressure changes its concentration more easily than the layer with high osmotic pressure. Button et al. propose a brush gel model that shows that when the mucus layer is heavily hydrated [4], its osmotic pressure drops sharply, so that the liquid from the airway surface enters the mucus layer first, while the PCL remains unchanged. Conversely, when airway dehydration occurs, the mucus layer is dehydrated first, increasing its concentration, increasing the osmotic pressure of the mucus layer, and the PCL layer is compressed under high pressure, causing the PCL to collapse. PCL is a network structure composed of various macromolecular substances, the size of which is not constant between grids, related to the height of the PCL layer [38]. When the airway surface fluid is overhydrated, resulting in the collapse of the PCL layer, the mesh size of the PCL layer is subsequently reduced; Assuming that the drug particles reach the mucus layer, the penetration of the drug particles in the lung mucosa is significantly reduced due to the reduction of the pores in the PCL grid. In addition, a series of changes in the composition and structure of the mucus layer in the pathological state, such as an imbalance in ion transport in the lung airways in patients with cystic fibrosis (CF), leads to a decrease in the volume of fluid on the surface of the airway, a significant increase in the viscoelasticity of the mucus, and impaired clearance of mucocilia [39][40][41][42]. In addition, under pathological conditions, the concentration of mucin, DNA, and actin increased significantly, significantly reducing the average size and size distribution of the mesh spacing, thereby severely hindering the transport of nanoparticles. Therefore, there is an urgent need for nanodelivery carriers capable of carrying drugs through dense mucus layers.
3. Nanoparticle-Mediated Effective Enhancement of Drug Retention and Penetration in the Airway Mucosa
Based on the barrier properties of airway mucus described earlier, researchers can design different strategies to enhance the penetration of drugs in the pulmonary mucosa according to their characteristics. Among them, nanoparticle formulations have significant advantages in improving cell penetration and therefore may be a promising approach to treating lung diseases [43]. However, when used as a transport carrier, it faces a double barrier of size filtration of the mucus layer and interaction filtration. Since the barrier properties of mucus change its behavior, it is necessary to design nanoparticles appropriately, such as changing the surface properties of nanoparticles (including particle surface functional groups and charge density, etc.), changing their particle size [44], etc. In addition, enlarging mucosal lattice voids by disrupting specific non-covalent interactions of mucogel is also an effective way to promote mucosal drug penetration. Table 1 summarizes the various types of nanoparticles that enhance mucus penetration and retention.
Table 1. Summary of nanoparticles for enhanced mucus penetration and retention.
| Nanoparticle type | Recipe details | outcome | reference |
|---|---|---|---|
|
|
|
[45] |
|
|
[46] | |
|
|
[47] | |
|
|
|
[48] |
|
|
[1] | |
|
|
|
[49] |
|
|
[50] | |
|
|
[51] | |
|
|
|
[52] |
|
|
[53] | |
|
|
|
[54] |
|
|
[55] | |
|
|
[56] | |
|
|
|
[57] |
|
|
[58] | |
|
|
|
[59] |
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15102457
References
- Osman, G.; Rodriguez, J.; Chan, S.Y.; Chisholm, J.; Duncan, G.; Kim, N.; Tatler, A.L.; Shakesheff, K.M.; Hanes, J.; Suk, J.S.; et al. PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J. Control. Release 2018, 285, 35–45.
- Bhat, P.G.; Flanagan, D.R.; Donovan, M.D. The limiting role of mucus in drug absorption: Drug permeation through mucus solution. Int. J. Pharm. 1995, 126, 179–187.
- Sears, P.R.; Davis, C.W.; Chua, M.; Sheehan, J.K. Mucociliary interactions and mucus dynamics in ciliated human bronchial epithelial cell cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L181–L186.
- Button, B.; Cai, L.H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012, 337, 937–941.
- Benam, K.H.; Vladar, E.K.; Janssen, W.J.; Evans, C.M. Mucociliary Defense: Emerging Cellular, Molecular, and Animal Models. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), S210–S215.
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell. Dev. Biol. 2018, 34, 189–215.
- Magnussen, H. Inhalation therapy for bronchial asthma: Strategies and targets. Curr. Opin. Pulm. Med. 2003, 9 (Suppl. S1), S3–S7.
- Duncan, G.A.; Jung, J.; Hanes, J.; Suk, J.S. The Mucus Barrier to Inhaled Gene Therapy. Mol. Ther. 2016, 24, 2043–2053.
- Cingolani, E.; Alqahtani, S.; Sadler, R.C.; Prime, D.; Stolnik, S.; Bosquillon, C. In vitro investigation on the impact of airway mucus on drug dissolution and absorption at the air-epithelium interface in the lungs. Eur. J. Pharm. Biopharm. 2019, 141, 210–220.
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013, 65, 1816–1827.
- Chakraborty, S.; Shukla, D.; Mishra, B.; Singh, S. Lipid—An emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. 2009, 73, 1–15.
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229.
- Chen, C.; Zhu, X.; Dou, Y.; Xu, J.; Zhang, J.; Fan, T.; Du, J.; Liu, K.; Deng, Y.; Zhao, L.; et al. Exendin-4 Loaded Nanoparticles with a Lipid Shell and Aqueous Core Containing Micelles for Enhanced Intestinal Absorption. J. Biomed. Nanotechnol. 2015, 11, 865–876.
- Peng, Q.; Zhang, Z.R.; Gong, T.; Chen, G.Q.; Sun, X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials 2012, 33, 1583–1588.
- Cortesi, R.; Campioni, M.; Ravani, L.; Drechsler, M.; Pinotti, M.; Esposito, E. Cationic lipid nanosystems as carriers for nucleic acids. New Biotechnol. 2014, 31, 44–54.
- das Neves, J.; Bahia, M.F.; Amiji, M.M.; Sarmento, B. Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opin. Drug Deliv. 2011, 8, 1085–1104.
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764.
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control. Release 2014, 192, 131–140.
- Bures, P.; Huang, Y.; Oral, E.; Peppas, N.A. Surface modifications and molecular imprinting of polymers in medical and pharmaceutical applications. J. Control. Release 2001, 72, 25–33.
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that “Slip” through the Human Mucus Barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729.
- Monaco, V.; Stefanini, C. Assessing the Tidal Volume through Wearables: A Scoping Review. Sensors 2021, 21, 4124.
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506.
- Pérez, B.F.; Méndez, G.A.; Lagos, R.A.; Vargas, M.S. Mucociliary clearance system in lung defense. Rev. Med. Chil. 2014, 142, 606–615.
- Meziu, E.; Koch, M.; Fleddermann, J.; Schwarzkopf, K.; Schneider, M.; Kraegeloh, A. Visualization of the structure of native human pulmonary mucus. Int. J. Pharm. 2021, 597, 120238.
- Button, B.; Okada, S.F.; Frederick, C.B.; Thelin, W.R.; Boucher, R.C. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci. Signal. 2013, 6, ra46.
- Falavigna, M.; Stein, P.C.; Flaten, G.E.; di Cagno, M.P. Impact of Mucin on Drug Diffusion: Development of a Straightforward in Vitro Method for the Determination of Drug Diffusivity in the Presence of Mucin. Pharmaceutics 2020, 12, 168.
- Song, D.; Cahn, D.; Duncan, G.A. Mucin Biopolymers and Their Barrier Function at Airway Surfaces. Langmuir 2020, 36, 12773–12783.
- Wan, F.; Herzberg, M.; Huang, Z.; Hassenkam, T.; Nielsen, H.M. A free-floating mucin layer to investigate the effect of the local microenvironment in lungs on mucin-nanoparticle interactions. Acta Biomater. 2020, 104, 115–123.
- Kirkham, S.; Sheehan, J.K.; Knight, D.; Richardson, P.S.; Thornton, D.J. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 2002, 361, 537–546.
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416.
- Wu, T.; Liao, W.; Wang, W.; Zhou, J.; Tan, W.; Xiang, W.; Zhang, J.; Guo, L.; Chen, T.; Ma, D.; et al. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr. Polym. 2018, 197, 403–413.
- Sands, M.; Kron, M.A.; Brown, R.B. Pentamidine: A review. Rev. Infect. Dis. 1985, 7, 625–634.
- Kadota, K.; Yanagawa, Y.; Tachikawa, T.; Deki, Y.; Uchiyama, H.; Shirakawa, Y.; Tozuka, Y. Development of porous particles using dextran as an excipient for enhanced deep lung delivery of rifampicin. Int. J. Pharm. 2019, 555, 280–290.
- Zheng, J.; Rubin, E.J.; Bifani, P.; Mathys, V.; Lim, V.; Au, M.; Jang, J.C.; Nam, J.; Dick, T.; Walker, J.R.; et al. para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis. J. Biol. Chem. 2013, 288, 28951.
- Gopal, P.; Gruber, G.; Dartois, V.; Dick, T. Pharmacological and Molecular Mechanisms Behind the Sterilizing Activity of Pyrazinamide. Trends Pharmacol. Sci. 2019, 40, 930–940.
- Murgia, X.; Pawelzyk, P.; Schaefer, U.F.; Wagner, C.; Willenbacher, N.; Lehr, C.M. Size-Limited Penetration of Nanoparticles into Porcine Respiratory Mucus after Aerosol Deposition. Biomacromolecules 2016, 17, 1536–1542.
- Webster, M.J.; Tarran, R. Slippery When Wet: Airway Surface Liquid Homeostasis and Mucus Hydration. Curr. Top Membr. 2018, 81, 293–335.
- Kaufman, H.; Arfken, G. Mathematical Methods for Physicists. Can. Math Bull. 1967, 10, 624.
- Butnarasu, C.; Caron, G.; Pacheco, D.P.; Petrini, P.; Visentin, S. Cystic Fibrosis Mucus Model to Design More Efficient Drug Therapies. Mol. Pharm. 2022, 19, 520–531.
- Dawson, M.; Wirtz, D.; Hanes, J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J. Biol. Chem. 2003, 278, 50393–50401.
- Suk, J.S.; Lai, S.K.; Wang, Y.Y.; Ensign, L.M.; Zeitlin, P.L.; Boyle, M.P.; Hanes, J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–2597.
- Pacheco, D.P.; Butnarasu, C.S.; Briatico Vangosa, F.; Pastorino, L.; Visai, L.; Visentin, S.; Petrini, P. Disassembling the complexity of mucus barriers to develop a fast screening tool for early drug discovery. J. Mater. Chem. B 2019, 7, 4940–4952.
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2021, 18, 595–606.
- Yu, M.; Wang, J.; Yang, Y.; Zhu, C.; Su, Q.; Guo, S.; Sun, J.; Gan, Y.; Shi, X.; Gao, H. Rotation-Facilitated Rapid Transport of Nanorods in Mucosal Tissues. Nano Lett. 2016, 16, 7176–7182.
- Kolte, A.; Patil, S.; Lesimple, P.; Hanrahan, J.W.; Misra, A. PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int. J. Pharm. 2017, 524, 382–396.
- Rao, K.S.V.K.; Zhong, Q.; Bielski, E.R.; da Rocha, S.R.P. Nanoparticles of pH-Responsive, PEG-Doxorubicin Conjugates: Interaction with an in Vitro Model of Lung Adenocarcinoma and Their Direct Formulation in Propellant-Based Portable Inhalers. Mol. Pharmaceut. 2017, 14, 3866–3878.
- Laffleur, F.; Hintzen, F.; Shahnaz, G.; Rahmat, D.; Leithner, K.; Bernkop-Schnurch, A. Development and in vitro evaluation of slippery nanoparticles for enhanced diffusion through native mucus. Nanomedicine 2014, 9, 387–396.
- Leal, J.; Dong, T.; Taylor, A.; Siegrist, E.; Gao, F.; Smyth, H.D.C.; Ghosh, D. Mucus-penetrating phage-displayed peptides for improved transport across a mucus-like model. Int. J. Pharm. 2018, 553, 57–64.
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; McMurtry, I.F.; Oka, M.; Nozik-Grayck, E.; Komatsu, M.; Ahsan, F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J. Control. Release 2013, 167, 189–199.
- Karamanidou, T.; Karidi, K.; Bourganis, V.; Kontonikola, K.; Kammona, O.; Kiparissides, C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 2015, 97 Pt A, 223–229.
- Conte, G.; Costabile, G.; Baldassi, D.; Rondelli, V.; Bassi, R.; Colombo, D.; Linardos, G.; Fiscarelli, E.V.; Sorrentino, R.; Miro, A.; et al. Hybrid Lipid/Polymer Nanoparticles to Tackle the Cystic Fibrosis Mucus Barrier in siRNA Delivery to the Lungs: Does PEGylation Make the Difference? ACS Appl. Mater. Interfaces 2022, 14, 7565–7578.
- Jang, S.; Park, J.W.; Cha, H.R.; Jung, S.Y.; Lee, J.E.; Jung, S.S.; Kim, J.O.; Kim, S.Y.; Lee, C.S.; Park, H.S. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int. J. Nanomed. 2012, 7, 1329–1343.
- Barreto, E.; Serra, M.F.; Dos Santos, R.V.; Dos Santos, C.E.; Hickmann, J.; Cotias, A.C.; Pão, C.R.; Trindade, S.G.; Schimidt, V.; Giacomelli, C.; et al. Local Administration of Gold Nanoparticles Prevents Pivotal Pathological Changes in Murine Models of Atopic Asthma. J. Biomed. Nanotechnol. 2015, 11, 1038–1050.
- Dunnhaupt, S.; Barthelmes, J.; Hombach, J.; Sakloetsakun, D.; Arkhipova, V.; Bernkop-Schnurch, A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int. J. Pharm. 2011, 408, 191–199.
- Pardeshi, C.V.; Agnihotri, V.V.; Patil, K.Y.; Pardeshi, S.R.; Surana, S.J. Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Int. J. Biol. Macromol. 2020, 165 Pt A, 445–459.
- du Plessis, L.H.; Kotze, A.F.; Junginger, H.E. Nasal and rectal delivery of insulin with chitosan and N-trimethyl chitosan chloride. Drug Deliv. 2010, 17, 399–407.
- Li, Y.; Han, M.; Liu, T.; Cun, D.; Fang, L.; Yang, M. Inhaled hyaluronic acid microparticles extended pulmonary retention and suppressed systemic exposure of a short-acting bronchodilator. Carbohydr. Polym. 2017, 172, 197–204.
- Liu, T.T.; Han, M.H.; Tian, F.; Cun, D.M.; Rantanen, J.; Yang, M.S. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: In vitro and in vivo evaluation. Carbohyd. Polym. 2018, 181, 1143–1152.
- Lababidi, N.; Montefusco-Pereira, C.V.; de Souza Carvalho-Wodarz, C.; Lehr, C.M.; Schneider, M. Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur. J. Pharm. Biopharm. 2020, 157, 200–210.
This entry is offline, you can click here to edit this entry!
