Inflammation and oxidative stress are essential components in a myriad of pathogenic entities that lead to metabolic and chronic diseases. Moreover, inflammation in its different phases is necessary for the initiation and maintenance of a healthy pregnancy. Therefore, an equilibrium between a necessary/pathologic level of inflammation and oxidative stress during pregnancy is needed to avoid disease development. High-density lipoproteins (HDL) are important for a healthy pregnancy and a good neonatal outcome. Their role in fetal development during challenging situations is vital for maintaining the equilibrium.
- inflammation
- oxidative stress
- pregnancy
- high-density lipoproteins
1. Introduction
2. Lipoproteins
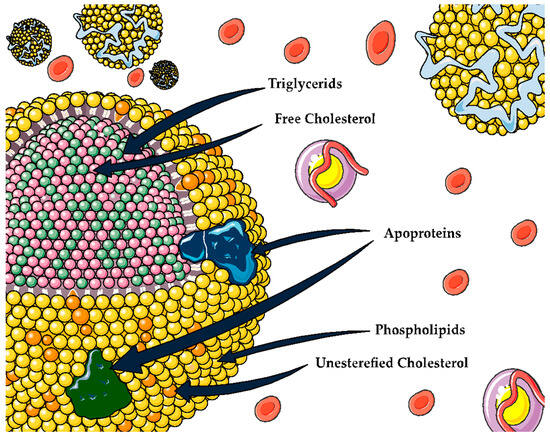
3. HDL
| HDL Function | Proteins and Lipids Associated with HDL Function | Ref. | |
|---|---|---|---|
| Reverse cholesterol transport | HDL promotes cholesterol efflux from various cell types. | ABCAI, ABCG1, SR-BI, cubilin, ApoE receptor | [14][15] |
| Removing excess cholesterol from lipid-laden macrophages is a crucial process in HDL-mediated vascular protection. | |||
| Oxidant | HDL has antioxidant properties whereby it can remove and inactivate lipid peroxides from LDL and cells. | PON 1, Apo AI, PAF-AH, LCAT, Apo M, S1P, phospholipids | [5] |
| Inflammation | Controlling the activation of monocytes, preventing macrophage migration, and inhibiting the oxidation of LDL by blocking the 12-lipoxygenase that produces lipid hydroperoxides and leads to the oxidation of the LDL | VCAM, ICAM, TNF-α, SAA, ceramides | [15] |
| Vascular function | Modulation of endothelial nitric oxide synthase (eNOS) expression, leading to increased nitric oxide (NO) production and vasodilation | ABCA1, SR-BI, S1PR, S1P, Apo M | [16][17][18] |
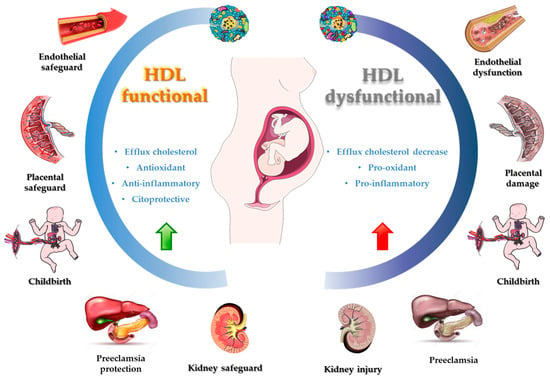
4. HDL Role and Upregulation Contribution in Inflammatory Processes and OS
5. Bioactive Compounds and HDL Functionality
This entry is adapted from the peer-reviewed paper 10.3390/antiox12101894
References
- Li, J.; Xu, Y.; Sun, Z.; Cai, Y.; Wang, B.; Zhang, M.; Ban, Y.; Hou, X.; Hao, Y.; Ouyang, Q.; et al. Differential Lipids in Pregnant Women with Subclinical Hypothyroidism and Their Correlation to the Pregnancy Outcomes. Sci. Rep. 2021, 11, 19689.
- Charlton, F.; Tooher, J.; Rye, K.A.; Hennessy, A. Cardiovascular Risk, Lipids and Pregnancy: Preeclampsia and the Risk of Later Life Cardiovascular Disease. Hear. Lung Circ. 2014, 23, 203–212.
- Adank, M.C.; Johansen, A.K.; Benschop, L.; Van Streun, S.P.; Smak Gregoor, A.M.; Øyri, L.K.L.; Mulder, M.T.; Steegers, E.A.P.; Holven, K.B.; Roeters van Lennep, J.E. Maternal Lipid Levels in Early Pregnancy as a Predictor of Childhood Lipid Levels: A Prospective Cohort Study. BMC Pregnancy Childbirth 2022, 22, 588.
- Estrada-Luna, D.; Ortiz-Rodriguez, M.A.; Medina-Briseño, L.; Carreón-Torres, E.; Izquierdo-Vega, J.A.; Sharma, A.; Cancino-Díaz, J.C.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules 2018, 23, 2730.
- Bausserman, L.L.; Herbert, P.N.; McAdam, K.P. Heterogeneity of Human Serum Amyloid A Proteins. J. Exp. Med. 1980, 52, 641–656.
- Davidson, W.S.; Shah, A.S.; Sexmith, H.; Gordon, S.M. The HDL Proteome Watch: Compilation of Studies Leads to New Insights on HDL Function. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2022, 1867, 159072.
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic Diversity of High Density Lipoproteins: Our Emerging Understanding of Its Importance in Lipid Transport and Beyond. J. Lipid Res. 2013, 54, 2575–2585.
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport: Basic Mechanisms and Their Roles in Vascular Health and Disease. Circ. Res. 2019, 124, 1505–1518.
- Cuchel, M.; Rader, D.J. Macrophage Reverse Cholesterol Transport: Key to the Regression of Atherosclerosis? Circulation 2006, 113, 2548–2555.
- Chen, C.; Khismatullin, D.B. Oxidized Low-Density Lipoprotein Contributes to Atherogenesis via Co-Activation of Macrophages and Mast Cells. PLoS ONE 2015, 10, e0123088.
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 601.
- Buendía-Abad, M.; García-Palencia, P.; de Pablos, L.M.; Alunda, J.M.; Osuna, A.; Martín-Hernández, R.; Higes, M. First Description of Lotmaria Passim and Crithidia Mellificae Haptomonad Stages in the Honeybee Hindgut. Int. J. Parasitol. 2022, 52, 65–75.
- Stadler, J.T.; Scharnagl, H.; Wadsack, C.; Marsche, G. Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring. Antioxidants 2023, 12, 795.
- Eren, E. High Density Lipoprotein and It’s Dysfunction. Open Biochem. J. 2012, 6, 78–93.
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High Density Lipoprotein as a Protective Factor against Coronary Heart Disease. The Framingham Study. Am. J. Med. 1977, 62, 707–714.
- Patanapirunhakit, P.; Karlsson, H.; Mulder, M.; Ljunggren, S.; Graham, D.; Freeman, D. Sphingolipids in HDL—Potential Markers for Adaptation to Pregnancy? Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2021, 1866, 158955.
- Luna-Luna, M.; Niesor, E.; Pérez-Méndez, Ó. HDL as Bidirectional Lipid Vectors: Time for New Paradigms. Biomedicines 2022, 10, 1180.
- Chen, R.; Chen, Q.; Zheng, J.; Zeng, Z.; Chen, M.; Li, L.; Zhang, S. Serum Amyloid Protein A in Inflammatory Bowel Disease: From Bench to Bedside. Cell Death Discov. 2023, 9, 154.
- Streese, L.; Habisch, H.; Deiseroth, A.; Carrard, J.; Infanger, D.; Schmidt-Trucksäss, A.; Madl, T.; Hanssen, H. Lipoprotein Subclasses Independently Contribute to Subclinical Variance of Microvascular and Macrovascular Health. Molecules 2022, 27, 4760.
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between Ethnicity and Obesity with High-Density Lipoprotein (HDL) Function and Subclass Distribution. Lipids Health Dis. 2016, 15, 92.
- Muñoz-Vega, M.; Massó, F.; Páez, A.; Vargas-Alarcón, G.; Coral-Vázquez, R.; Mas-Oliva, J.; Carreón-Torres, E.; Pérez-Méndez, Ó. Hdl-Mediated Lipid Influx to Endothelial Cells Contributes to Regulating Intercellular Adhesion Molecule (Icam)-1 Expression and Enos Phosphorylation. Int. J. Mol. Sci. 2018, 19, 3394.
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative Activity of High-Density Lipoprotein (HDL): Mechanistic Insights into Potential Clinical Benefit. BBA Clin. 2017, 8, 66–77.
- Jayaraman, S.; Haupt, C.; Gursky, O. Thermal Transitions in Serum Amyloid A in Solution and on the Lipid: Implications for Structure and Stability of Acute-Phase HDL. J. Lipid Res. 2015, 56, 1531–1542.
- Göçmen, A.Y.; Şahin, E.; Semiz, E.; Gümüslü, S. Is Elevated Serum Ceruloplasmin Level Associated with Increased Risk of Coronary Artery Disease? Can. J. Cardiol. 2008, 24, 209–212.
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of Infection and Inflammation on Lipid and Lipoprotein Metabolism: Mechanisms and Consequences to the Host. J. Lipid Res. 2004, 45, 1169–1196.
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2016, 13, 48–60.
- Zafaranieh, S.; Stadler, J.T.; Pammer, A.; Marsche, G.; van Poppel, M.N.M.; Desoye, G. The Association of Physical Activity and Sedentary Behavior with Maternal and Cord Blood Anti-Oxidative Capacity and HDL Functionality: Findings of DALI Study. Antioxidants 2023, 12, 827.
- Schliefsteiner, C.; Hirschmugl, B.; Kopp, S.; Curcic, S.; Bernhart, E.M.; Marsche, G.; Lang, U.; Desoye, G.; Wadsack, C. Maternal Gestational Diabetes Mellitus Increases Placental and Foetal Lipoprotein-Associated Phospholipase A2 Which Might Exert Protective Functions against Oxidative Stress. Sci. Rep. 2017, 7, 12628.
- León-Reyes, G.; Espino, Y.; Sosa, S.; Medina-Navarro, R.; Guzmán-Grenfell, A.M.; Medina-Urrutia, A.X.; Fuentes-García, S.; Hicks, G.J.J.; Torres-Ramos, Y.D. Oxidative Modifications of Foetal LDL-c and HDL-c Lipoproteins in Preeclampsia. Lipids Health Dis. 2018, 17, 110.
- Jayalekshmi, V.S.; Jaikumar, V.S.; Mehra, P.; Thulaseedharan, T.; Vinod, V.M.; Ramachandran, S. Differential Expression of Lipid Metabolic Genes in Hypercholesterolemic Rabbit Placenta Predisposes the Offspring to Develop Atherosclerosis in Early Adulthood. Life Sci. 2023, 327, 121823.
- Rodríguez-Hernández, H.; Simental-Mendía, L.E. The Triglycerides and Glucose Index Is Highly Associated with Non-Alcoholic Fatty Liver Disease in Overweight and Obese Women. Ir. J. Med. Sci. 2023, 2023, 11845.
- Zhou, Y.; Peng, H.; Xu, H.; Li, J.; Golovko, M.; Cheng, H.; Lynch, E.C.; Liu, L.; McCauley, N.; Kennedy, L.; et al. Maternal Diet Intervention before Pregnancy Primes Offspring Lipid Metabolism in Liver. Lab. Investig. 2020, 100, 553–569.
- Mocciaro, G.; Allison, M.; Jenkins, B.; Azzu, V.; Huang-Doran, I.; Herrera-Marcos, L.V.; Hall, Z.; Murgia, A.; Susan, D.; Frontini, M.; et al. Non-Alcoholic Fatty Liver Disease Is Characterised by a Reduced Polyunsaturated Fatty Acid Transport via Free Fatty Acids and High-Density Lipoproteins (HDL). Mol. Metab. 2023, 73, 101728.
- Pelczyńska, M.; Moszak, M.; Wesołek, A.; Bogdański, P. The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation. Antioxidants 2023, 12, 1232.
- Pathikkal, A.; Puthusseri, B.; Divya, P.; Rudrappa, S.; Chauhan, V.S. Folate Derivatives, 5-Methyltetrahydrofolate and 10-Formyltetrahydrofolate, Protect BEAS-2B Cells from High Glucose–Induced Oxidative Stress and Inflammation. Vitr. Cell. Dev. Biol.—Anim. 2022, 58, 419–428.
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 803294.
- Delmastro-Greenwood, M.; Freeman, B.A.; Wendell, S.G. Redox-Dependent Anti-Inflammatory Signaling Actions of Unsaturated Fatty Acids. Annu. Rev. Physiol. 2014, 76, 79–105.
- Gong, S.; Ji, X.; Su, J.; Wang, Y.; Yan, X.; Wang, G.; Xiao, B.; Dong, H.; Xiang, X.; Liu, S. Yeast Fermentate Prebiotic Ameliorates Allergic Asthma, Associating with Inhibiting Inflammation and Reducing Oxidative Stress Level through Suppressing Autophagy. Mediat. Inflamm. 2021, 2021, 4080935.
- Dai, Y.; Quan, J.; Xiong, L.; Luo, Y.; Yi, B. Probiotics Improve Renal Function, Glucose, Lipids, Inflammation and Oxidative Stress in Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Ren. Fail. 2022, 44, 862–880.
- Kelesidis, T.; Jackson, N.; Mccomsey, G.A.; Wang, X.; Elashoff, D.; Dube, M.P.; Brown, T.T.; Yang, O.O.; Stein, J.H.; Currier, J.S. Oxidized Lipoproteins Are Associated with Markers of Inflammation and Immune Activation in HIV-1 Infection. AIDS 2016, 30, 2625–2633.
- Vergès, B. Dyslipidemia in Type 1 Diabetes: A Masked Danger. Trends Endocrinol. Metab. 2020, 31, 422–434.
- Ganjali, S.; Dallinga-Thie, G.M.; Simental-Mendía, L.E.; Banach, M.; Pirro, M.; Sahebkar, A. HDL Functionality in Type 1 Diabetes. Atherosclerosis 2017, 267, 99–109.
- Stadler, J.T.; Marsche, G. Dietary Strategies to Improve Cardiovascular Health: Focus on Increasing High-Density Lipoprotein Functionality. Front. Nutr. 2021, 8, 1–16.
- Cardner, M.; Yalcinkaya, M.; Goetze, S.; Luca, E.; Balaz, M.; Hunjadi, M.; Hartung, J.; Shemet, A.; Kränkel, N.; Radosavljevic, S.; et al. Structure-Function Relationships of HDL in Diabetes and Coronary Heart Disease. JCI Insight 2020, 5, 1–18.
- Luna-Castillo, K.P.; Lin, S.; Muñoz-Valle, J.F.; Vizmanos, B.; López-Quintero, A.; Márquez-Sandoval, F. Functional Food and Bioactive Compounds on the Modulation of the Functionality of Hdl-c: A Narrative Review. Nutrients 2021, 13, 1165.
- Vaisar, T.; Kanter, J.E.; Wimberger, J.; Irwin, A.D.; Gauthier, J.; Wolfson, E.; Bahnam, V.; Wu, I.H.; Shah, H.; Keenan, H.A.; et al. High Concentration of Medium-Sized HDL Particles and Enrichment in HDL Paraoxonase 1 Associate with Protection from Vascular Complications in People with Long-Standing Type 1 Diabetes. Diabetes Care 2020, 43, 178–186.
- Gu, X.; Huang, Y.; Levison, B.S.; Gerstenecker, G.; DiDonato, A.J.; Hazen, L.B.; Lee, J.; Gogonea, V.; DiDonato, J.A.; Hazen, S.L. Identification of Critical Paraoxonase 1 Residues Involved in High Density Lipoprotein Interaction. J. Biol. Chem. 2016, 291, 1890–1904.
- Dorantes-Morales, A.; Estrada-Luna, D.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Luna-Luna, M.; Flores-Castillo, C.; Vargas-Alarcón, G.; Fragoso, J.M.; Pérez-Méndez, Ó.; Carreón-Torres, E.; et al. Microencapsulated Pomegranate Modifies the Composition and Function of High-Density. Molecules 2020, 25, 3297.
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation 2017, 135, 633–643.
- Hernáez, Á.; Fernández-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive Oil Polyphenols Enhance High-Density Lipoprotein Function in Humans: A Randomized Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119.
- Sola, R.; Valls, R.M.; Puzo, J.; Calabuig, J.R.; Brea, A.; Pedret, A.; Morinã, D.; Villar, J.; Millán, J.; Anguera, A. Effects of Poly-Bioactive Compounds on Lipid Profile and Body Weight in a Moderately Hypercholesterolemic Population with Low Cardiovascular Disease Risk: A Multicenter Randomized Trial. PLoS ONE 2014, 9, e101978.
- Bub, A.; Malpuech-Brugère, C.; Orfila, C.; Amat, J.; Arianna, A.; Blot, A.; Di Nunzio, M.; Holmes, M.; Kertész, Z.; Marshall, L.; et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients 2019, 11, 1814.
- Muralidharan, J.; Papandreou, C.; Soria-Florido, M.T.; Sala-Vila, A.; Blanchart, G.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Ros, E.; Ruiz-Canela, M.; et al. Cross-Sectional Associations between HDL Structure or Function, Cell Membrane Fatty Acid Composition, and Inflammation in Elderly Adults. J. Nutr. 2022, 152, 789–795.
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Perez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated Pomegranate Reverts High-Density Lipoprotein (HDL)-Induced Endothelial Dysfunction and Reduces Postprandial Triglyceridemia in Women with Acute Coronary Syndrome. Nutrients 2019, 11, 1710.
