Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A retinal prosthesis, also known as a bionic eye, is a device that can be implanted to partially restore vision in patients with retinal diseases that have resulted in the loss of photoreceptors (e.g., age-related macular degeneration and retinitis pigmentosa). There have been major breakthroughs in retinal prosthesis technology, with the creation of numerous types of implants, including epiretinal, subretinal, and suprachoroidal sensors. These devices can stimulate the remaining cells in the retina with electric signals to create a visual sensation.
- retinal prosthesis
- vision restoration
- retinal disease
- ophthalmology
- retinal prostheses
1. Introduction
1.1. Overview of Retinal Prostheses
In recent years, the field of retinal prosthetics has gained considerable attention due to its potential to restore some useful vision to individuals suffering from retinal diseases that have resulted in the loss of photoreceptors, such as age-related macular degeneration and retinitis pigmentosa. These conditions can lead to significant vision loss, severely impacting patients’ quality of life and ability to perform daily tasks. Retinal prostheses, also known as bionic eyes, offer a promising solution by stimulating the remaining retinal cells to produce visual sensations.
The development of retinal prosthetic devices has been marked by significant technological advancements, including the introduction of various implant designs such as epiretinal, subretinal, and suprachoroidal sensors. These innovations have expanded the range of possibilities for enhancing patients’ visual acuity and improving the overall functionality of retinal prosthetic devices.
1.2. Overview of Retinal Structure and Function
The retina, a thin and transparent structure, embryologically originates from the inner and outer layers of the optic cup. It comprises 10 distinct layers, each playing a crucial role in the visual processing pathway. In a cross section, these layers can be identified from the inner to the outer retina as follows (Figure 1).
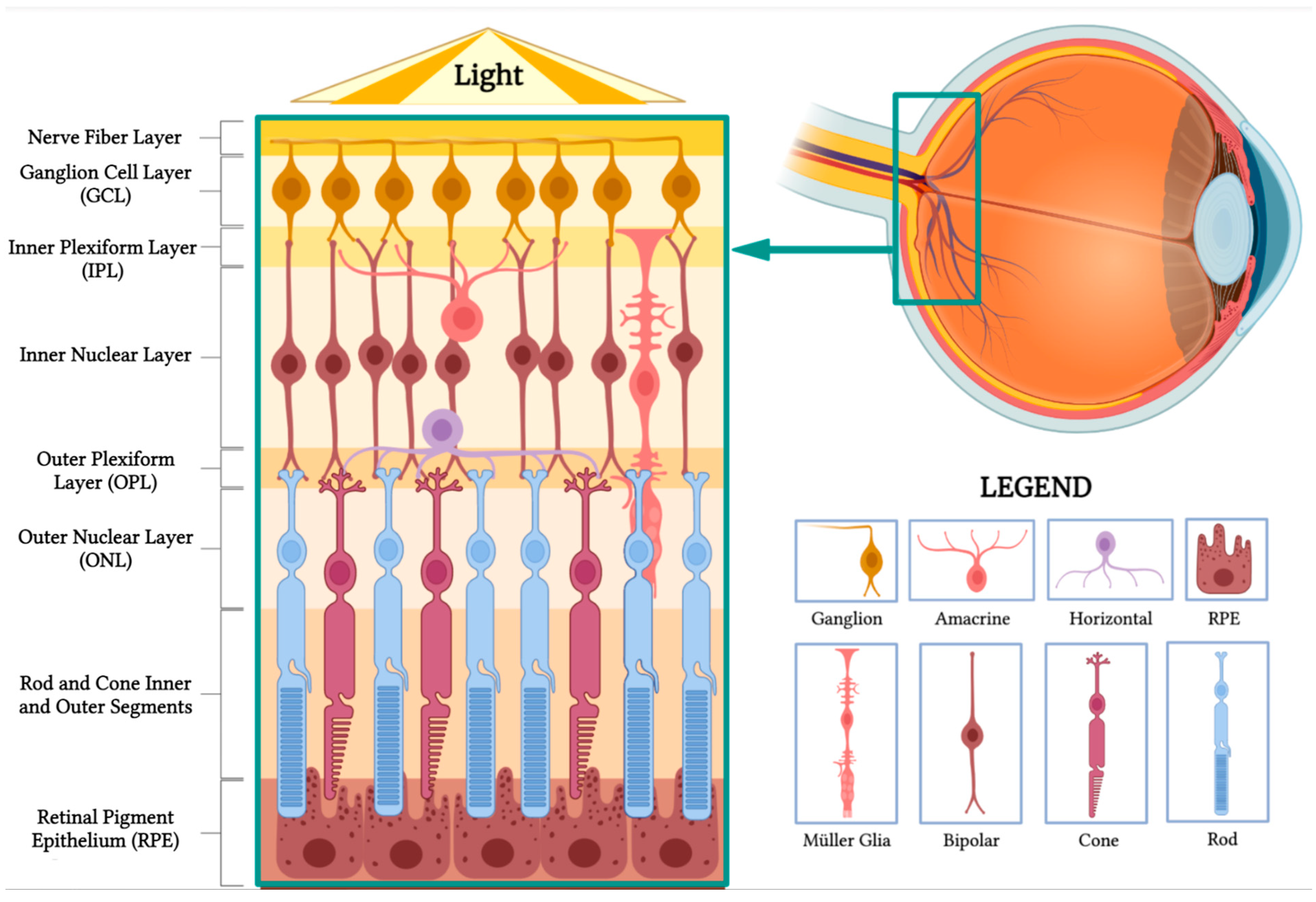
Figure 1. Retinal anatomy. The illustration highlights the different layers of the retina and its main cell types. (BioRender, https://app.biorender.com/, accessed on 16 February 2023).
-
Nerve fiber layer: This layer contains the axons of retinal ganglion cells that coalesce to form the optic nerve.
-
Ganglion cell layer: This layer is composed of the cell bodies of retinal ganglion cells, which transmit visual information to the brain.
-
Inner plexiform layer: This layer consists of the synapses between bipolar and ganglion cells, facilitating signal processing and integration.
-
Inner nuclear layer: This layer houses the cell bodies of bipolar, horizontal, and amacrine cells, which play essential roles in processing and transmitting visual information.
-
Outer plexiform layer: This layer contains synapses between photoreceptors, bipolar cells, and horizontal cells, allowing for the initial processing of visual signals.
-
Outer nuclear layer: This layer is made up of the cell bodies of rod and cone photoreceptors, which are responsible for capturing and converting light into neural signals.
-
Rod and cone inner and outer segments: These segments are part of the photoreceptor cells, which include rods and cones. The inner segments contain vital cellular components, such as mitochondria, while the outer segments contain stacked discs rich in photopigments, which are essential for absorbing light and initiating the phototransduction cascade to produce neuronal signals.
-
Retinal pigment epithelium (RPE): This is the outermost layer of the retina, located just beneath the photoreceptor cells. The RPE has several crucial functions in visual processing. Its cells absorb stray light, preventing light scatter and enhancing visual acuity. They also play a vital role in recycling photopigments and shuttling nutrients to the photoreceptors. Additionally, they facilitate the transport of metabolic waste products from the photoreceptors to the choroidal blood supply, thereby helping to maintain the health of the photoreceptor cells.
Horizontal cells make synaptic connections with rod spherules and cone pedicles, while bipolar cells are vertically oriented, synapsing with either rod or cone synaptic bodies. Their axons make synaptic contact with ganglion cells and amacrine cells in the inner plexiform layer. The axons of ganglion cells form the nerve fiber layer and later the optic nerve, containing over one million optic nerve fibers [1].
1.3. Overview of Retinal Physiology and Pathology
Retinal prostheses aim to restore vision in degenerate eyes by replacing the function of the photoreceptors. In the normal eye, the photoreceptors in the outer layers of the retina contain light-sensitive pigments that trigger the phototransduction cascade, generating neuronal signals upon light stimuli. These signals are processed by a complex network of neurons within the middle layers of the retina before reaching retinal ganglion cells (RGCs) in the inner layers. Axonal processes from RGCs form the optic nerve, transmitting light-evoked neuronal signals to the visual cortex. However, in congenital retinal dystrophies such as retinitis pigmentosa, the photoreceptors in the outer layers are gradually lost, causing progressive visual loss, while the inner retinal layers, including RGCs and bipolar cells, are partially spared [2].
Theoretically, vision restoration could be achieved by creating retinal prostheses that receive and process incoming light, transmitting the information as electrical impulses to the remaining inner retinal layers for visual function. However, the retina’s complex physiology poses significant challenges for retinal prostheses, as devices must replicate intricate retinal processing. Currently, electrical or photovoltaic stimulation is provided in a relatively unspecified manner, simultaneously activating multiple cell types. The challenge lies in developing more selective stimulation to optimize device resolution and patient outcomes.
2. Principles of Electronic Retinal Prostheses
At the intersection of science and engineering, the field of visual prosthetics is driven by an ambition–to restore vision to the blind. The epitome of success in the field would be to create vision comparable to that of a healthy retina. Natural vision is made possible through complex, highly orchestrated, temporally synchronized electrical signals fired by retinal ganglion cells [3]. This, then, becomes the goal of retinal prosthesis–to receive a light stimulus, convert it to an electric pulse, and deliver that pulse to the retina through a microelectrode array. Through this electric signal, prostheses aim to stimulate the structures of the retina that remain capable of conveying signals to the optic nerve, which transmits them to the brain for interpretation.
Mainly, two main mechanisms were described that allow prostheses to provide electrical stimulation to retinal cells [4]. The first mechanism works through external light detection and internal impulse delivery. In this mechanism, a microelectrode array is intraocularly implanted. This electrode array cannot convert light signals to electrical impulses by itself. Thus, it depends on an external camera system, such as one mounted on a set of glasses, to receive an image from the surroundings. That image is then processed (i.e., the image is converted to electrical impulses) and sent to a periocular implant (Figure 2). Finally, the periocular implant sends the electrical signal to the implanted microelectrode via cables. The microelectrode array then conveys it to the retinal cells. An example of one such system is the ARGUS II prosthetic (Figure 2 and Figure 3) [5]. In the second mechanism, a photodiode electrode array that can act directly as the image receiver is implanted. This photodiode electrode is able to directly receive light energy and transduce it to an electrical signal (Figure 4) [6]. The light-induced signal can then be used to stimulate retinal cells. These photodiode-based systems aim to replace the lost photoreceptors of the retina, and they can often fulfill their function without having processing units or external cameras. In some cases, however, external components including external power were used to amplify the light waves and produce suitably strengthened impulses [7]. Generally, prostheses using the second mechanism are activated directly by light and can depend on photovoltaic processes, such as creating a voltage drop, to produce an electric field which can then be used to stimulate target cells [8]. Photodiode systems have multiple advantages. (1) Due to their photovoltaic process, photodiode-based prostheses can avoid the use of implanted cables. Consequently, the surgical implantation tends to be less complex for photodiode-based prostheses than for implants requiring cable connections. (2) These systems can directly transduce light stimuli to electric signals and are, thus, often functional without the use of an external camera. Eliminating the need for an external camera maintains the link between eye movements and visual stimuli [9].
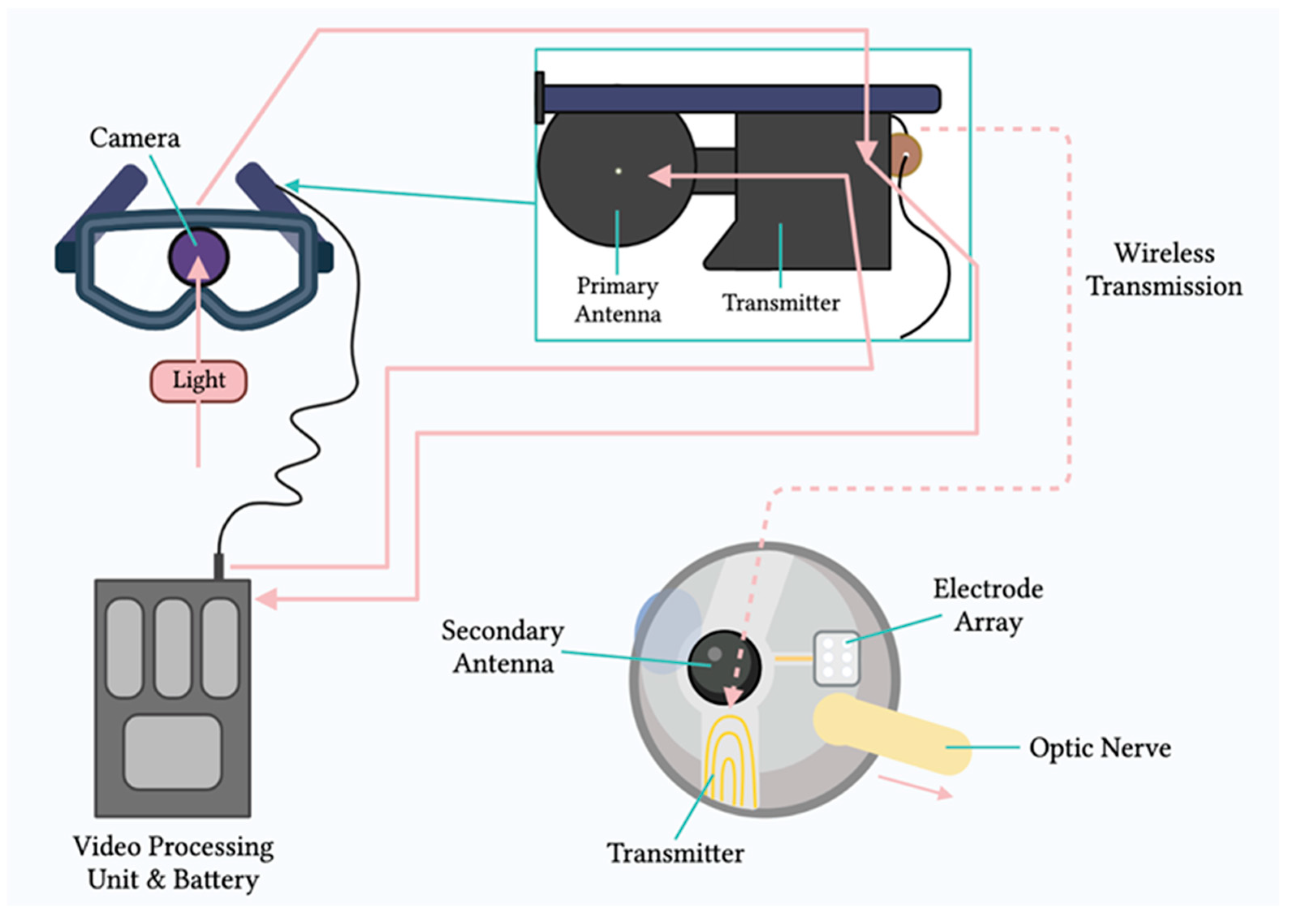
Figure 2. Components of the ARGUS II system. Schematic representation of the main components of the ARGUS II retinal prosthesis system, including the camera, video processing unit, and electrode array.
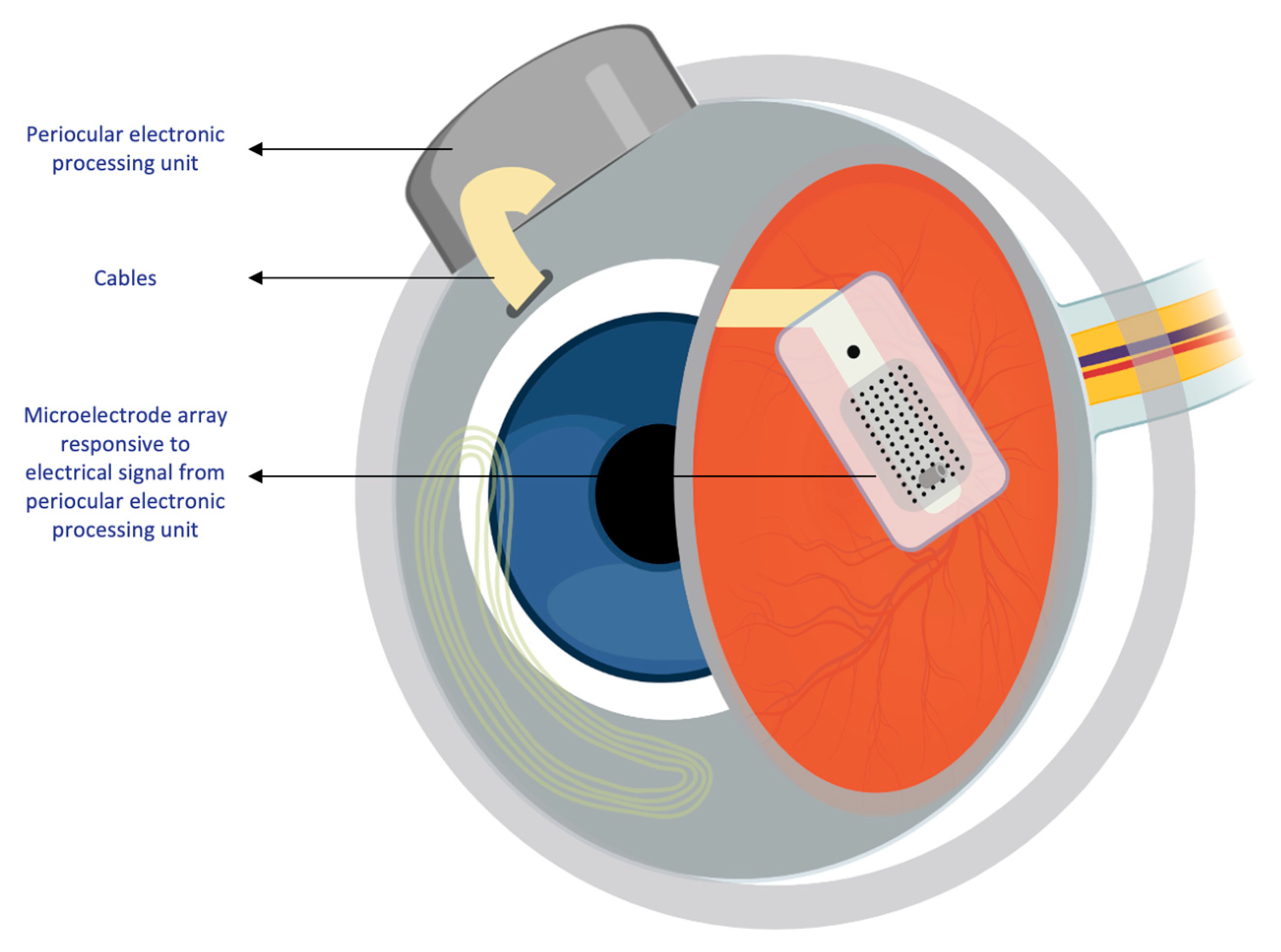
Figure 3. Intraocular components of the ARGUS II system. Diagram showing the internal components of the ARGUS II retinal prosthesis system that are implanted within the eye.
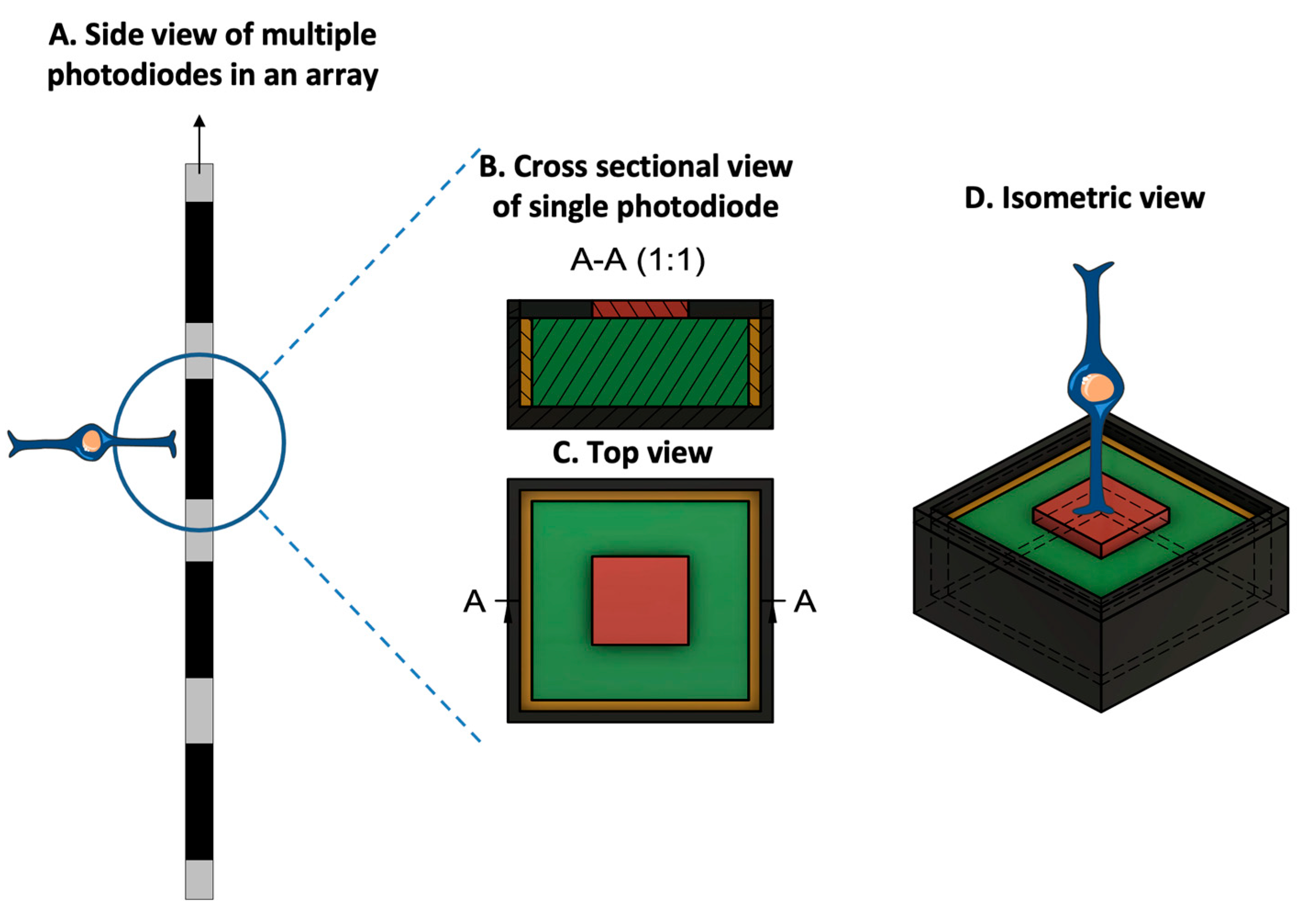
Figure 4. Prototypical design configuration of a photodiode, consisting of an inner electrode (red), a photodiode (green), an insulating layer (yellow), and an outer ground electrode (dark gray). (A. Side view of multiple photodiodes in an array. B. Cross-sectional view of the photodiode at position A-A, at the location specified in the top view. C. Top-orientation view of photodiode. D. Isometric view of the photodiode with a bipolar cell in close relation to the inner electrode. Due to the voltage drop between the inner and outer electrodes, the photodiode generates an electric field that can be used for cell stimulation. (Figure 4 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
Regardless of the mechanism of action, however–whether the prosthesis has internal non-light-sensitive electrodes or photodiodes–both systems must fulfill common engineering design criteria.
3. Engineering of Retinal Prostheses
The performance of retinal prostheses is dependent on a few outcomes. Fulfilling these outcomes is important to drive their adoption in the market and by patients. Each of the outcomes for retinal prostheses is closely related to an engineering design challenge, namely, a constraint (Figure 5).
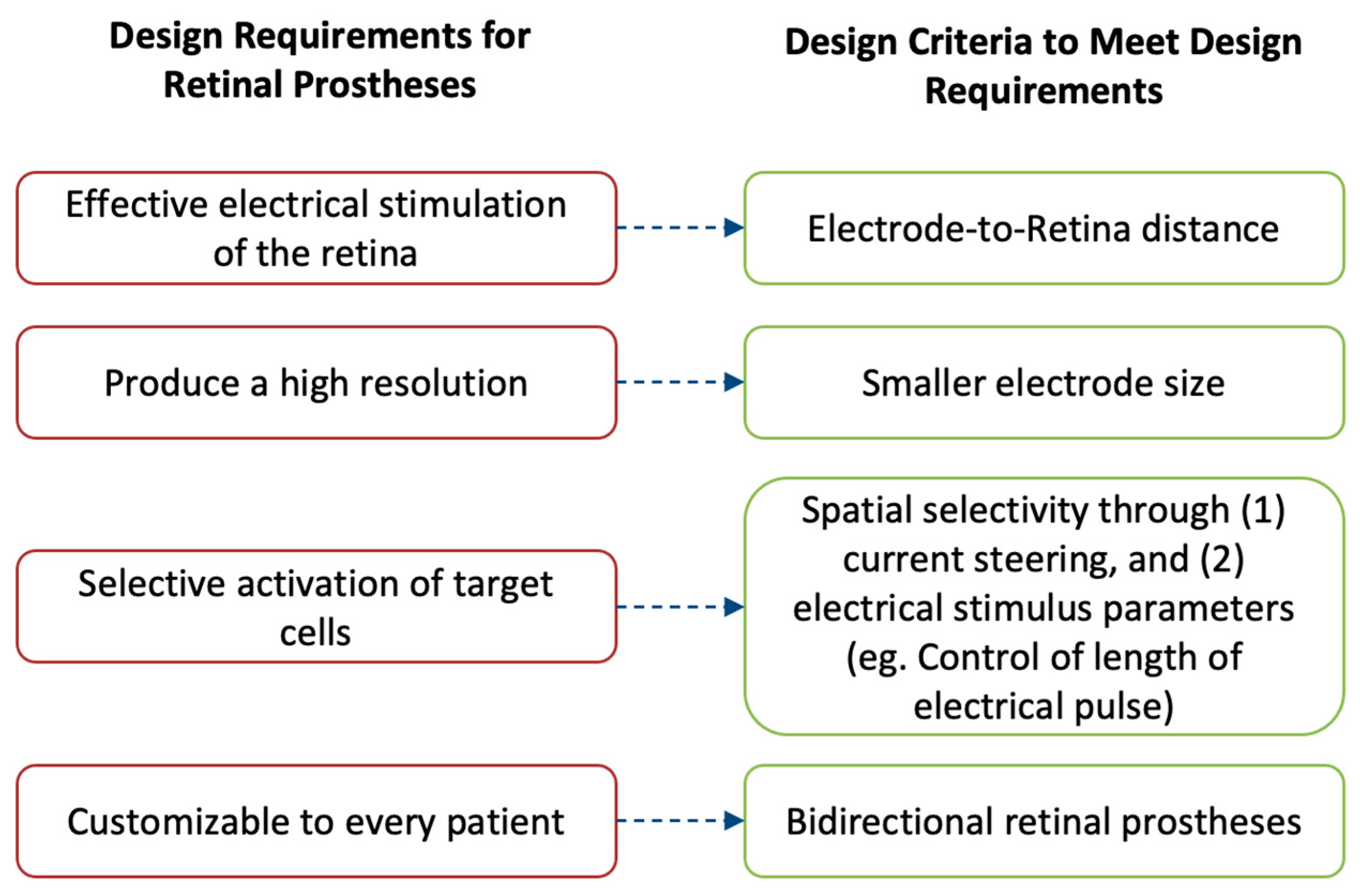
Figure 5. The performance of retinal prostheses is dependent on a few outcomes. Fulfilling these outcomes is important to drive their adoption in the market and by patients. These outcomes are (1) to provide effective electrical stimulation of the retina; (2) to produce a high-resolution image; (3) to selectively activate desired retinal cells, thereby avoiding image distortion; and (4) to be customizable for different patients. To achieve these requirements, retinal prostheses are designed with four main design criteria: (1) the electrode-to-retina distance, (2) having smaller electrode size, (3) implementing techniques to produce spatial selectivity, and (4) implementing bidirectional systems.
Firstly, retinal prostheses must provide effective electrical stimulation to the retina to enable formation of light percepts. To achieve this outcome, the microelectrode array of prostheses must directly contact the retina for successful stimulation. Even small separations between the microelectrode array and the retina can reduce a prosthetic’s efficacy.
Secondly, it is important for retinal prostheses to produce a high-resolution image that can then be interpreted by the recipient. To attain higher resolution, one main consideration is to minimize the size of the individual microelectrodes on the array such that each electrode can stimulate a single retinal cell and maximize the resolution limit of prostheses. However, there are technical challenges with such miniaturizing of microelectrodes. These challenges are described here, along with some of the solutions proposed to overcome them.
Thirdly, another design consideration of retinal prostheses is their ability to selectively target the desired cells in the retina. Different retinal cells produce different perceptions that directly impact the patient outcomes.
Finally, an important design consideration is a prosthetic’s ability to be customizable to every recipient’s individual needs. To achieve such an outcome, prostheses must be able to both convey electrical pulses and be able to record the impact of such stimulation on the retina. Thus, a description of the incorporation of bidirectional retinal prostheses is given.
It is mainly these design criteria that guide the manufacturing of retinal prostheses and dictate their future market adoption. Thus, each of the criteria is described in detail to allow the reader to understand the challenges, learn about recent advancements, and contemplate the prospects of retinal prostheses.
3.1. Electrode–Retina (ER) Topographical Alignment
MEAs directly interface with the retina to deliver electrical stimuli. Signals delivered by MEAs are either directly or indirectly received by the retinal ganglion cells (RGCs). In the case of epiretinal prostheses, the electrical signals are directly received, since the prostheses are in direct contact with the RGCs. In the case of subretinal/suprachoroidal prostheses, however, the signals are first received by cells in the posterior retina, which then convey them to the RGCs through the physiologic pathways. The received signal is then conveyed by the RGCs, through the optic nerve, to the brain, where they are interpreted as images [10]. The transmission of an electric stimulus to retinal neurons requires a close topographical fit between the MEA of the retinal prostheses and the retinal tissue [11]. The lack of conformity between implants and retinal tissue can cause gaps up to several hundred micrometers, which result in an impaired or lost signal in these areas and, consequently, impact the effectiveness of the device (Figure 6, A) [12]. Thus, researchers proposed a variety of solutions to overcome this topographical misalignment that can occur between the retina and the implant.
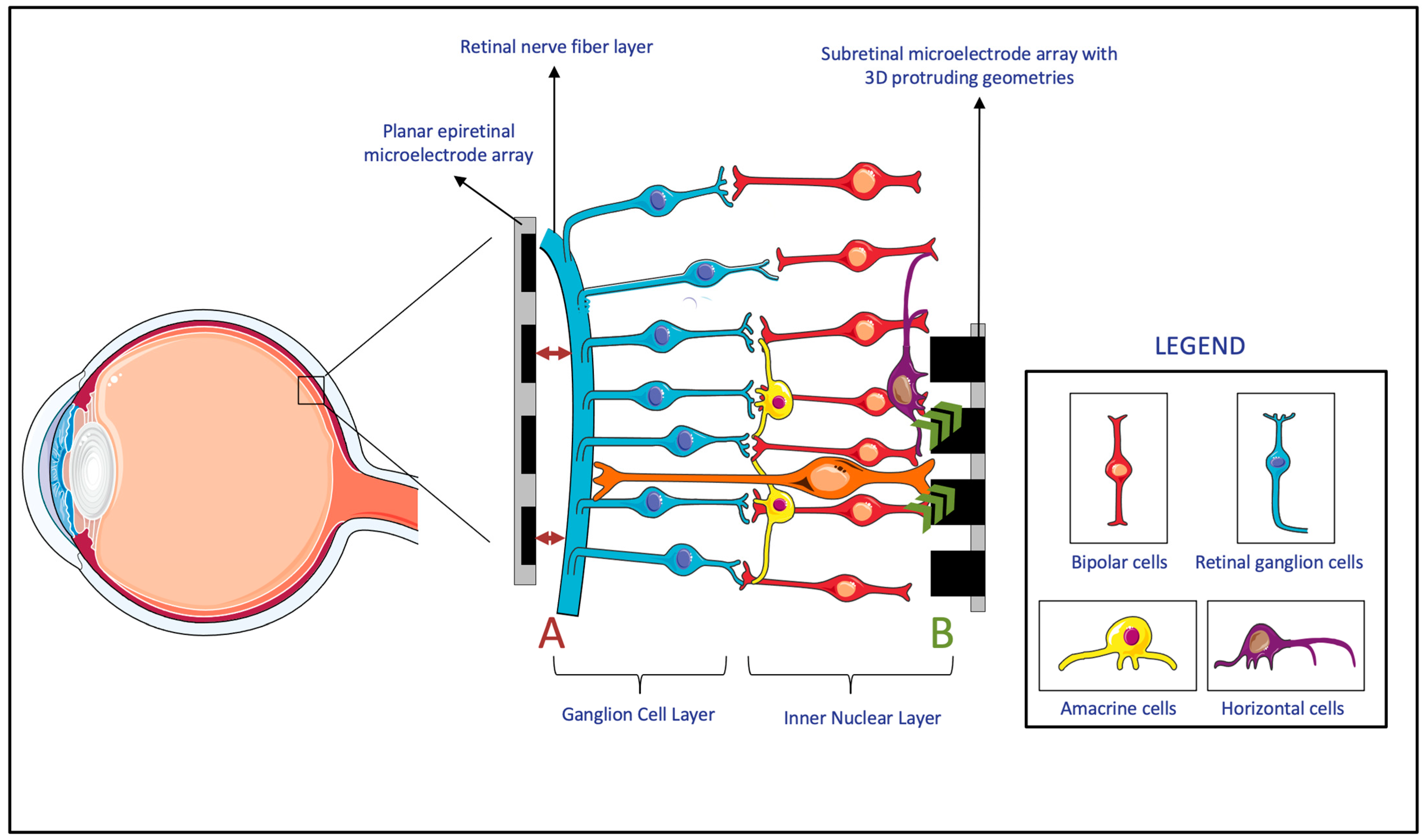
Figure 6. (A) The lack of the topographical alignment between a planar epiretinal microelectrode array and the retinal ganglion cells. (B) Migration and integration of the cells in the Inner Nuclear Layer to 3D protruding geometries in subretinal microelectrode array. (Figure 6 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
Firstly, many researchers developed MEAs that incorporate three-dimensional (3D) geometries. Within the retina, experiments showed that cells of the inner neuron layer (INL) can migrate and integrate with 3D geometry MEAs (Figure 6, B) [13]. Since subretinal prostheses are located in the INL, they are the ones that benefit the most from such integration of cells into the 3D geometry of MEAs. It was shown that both the protruding and recessed 3D geometries of subretinal implants benefit from this retinal migration, which reduces the separation distance between the INL and the implanted electrodes [13][14][15][16][17]. However, while this advantage applies to subretinal prostheses, both epiretinal and suprachoroidal implants were reported to have a gradually increasing ER distance over time. This observed phenomenon was attributable to fibrosis and to evoked inflammatory responses [18]. Since this time-based change in ER distance is particularly evident in epiretinal and suprachoroidal prostheses, these prostheses had more studies monitor this measurement, compared to studies of subretinal implants, in which device trials never measured the ER distance [18]. It is also worth noting that while it might be possible for retinal migration to decrease the ER distance in subretinal implants, epiretinal implants require mechanical pressure to achieve close proximity to the MEA [17]. All in all, due to the benefits of incorporating 3D geometries, these MEA designs were considered for subretinal devices. However, epiretinal and suprachoroidal MEAs rely on other methods that are more effective with their implant location.
Secondly, to decrease the electrode–retina distance, researchers considered integrating pneumatic cavities that enable dynamic, real-time control of the electrode position. In MEAs, pneumatic cavities can be placed under the electrodes [11], and, by adjusting the pneumatic pressure, it then becomes possible to change the electrode position and reduce the distance to the retinal surface. Like the proposed design of pneumatic systems, hydraulic systems can also be tested for incorporation, especially in epiretinal devices, to improve the topographical alignment.
Thirdly, researchers developed flexible MEA substrates that can be used to achieve topographical alignment. Beyond their use in brain neuron stimulation, flexible probes were suggested for use in epiretinal electrode arrays [19][20]. Such flexible designs can better fit the surface topographies and can, therefore, permit the creation of larger and higher-density devices, which can extend over the curvature of the retina. These flexible designs were also shown to be safe and effective [21][22]. Finally, the flexibility of these MEAs was considered beneficial in reducing the acute insertion footprint of the electrodes, an outcome often measured by retinal cell viability after the implantation [23]. However, while these MEAs are advantageous in many facets, including that they provide better topographical alignment, it can still be challenging for these microelectrode arrays to fill smaller gaps and sharp corners [11].
All in all, as an important engineering consideration, a variety of solutions were suggested to decrease the electrode–retina distance in the different types of retinal prostheses. The advantages and disadvantages of each of the solutions should be weighed, and, based on implant location, the appropriate feature should be incorporated.
3.2. Electrode Size and Material, Charge Density, and Resolution Limit
While most clinical results from human-implanted prostheses showed positive outcomes, the visual resolution obtained from prostheses has, nevertheless, remained limited. The recognition of simple objects and facial identification can still be challenging [24]. Similarly, the restoration of visual acuity in patients with retinal prosthesis has been, despite pioneering success in human trials, quite limited. Clinically, to describe visual acuity, the Snellen scale is often used. The scale has a large range, but specific acuities are defined—“normal” vision is defined as 20/20, and an acuity of 20/200 is defined as legally blind. The recent clinical trials of the PRIMA system, which is described in further detail in the succeeding sections, reported the best visual acuities of retinal prostheses yet, in the range of 20/460–20/565 [25]. While these acuities remain within the definition of legal blindness, a closer look reveals that there is a close match between the prosthetic acuity and the fundamental sampling limit set by the pixel size [26]. Quantitatively, it was reported that the visual acuity corresponded to 1.17 ± 0.13 of the pixel size [27]. From this observation, it can be inferred that the pixel size of the microelectrodes in the prosthesis was limiting the stimulation of adjacent points on the retina and, thereby, limiting acuity. This close match between the acuity and the fundamental sampling limit of the prosthetic consequently indicates that smaller pixels may provide higher resolution (Figure 7) [26].

Figure 7. Demonstrating the impact of smaller electrodes. In comparison to the 15 × 15 grid (A), the 5 × 5 grid (B) produces a lower image resolution and enables less object recognition. Additionally, since each square in the grid represents the size of an electrode, having larger electrodes correlates to larger activation of retinal cells—i.e., less selective activation of desired, target retinal cells.
In addition to this clinical observation, physiologically, to restore natural vision, retinal prostheses should ideally stimulate individual retinal neurons [10]. There are more than 1.5 million RGCs in the human retina, of which the largest soma has a diameter of about 30 μm [28]. In comparison, the smallest electrode size of the retinal prostheses that have been applied in clinical trials is the 50 × 50 μm electrode of the Alpha IMS system [29]. Therefore, both given the clinical results of the PRIMA system and to emulate physiological processes, it is logical to develop MEAs with smaller, more densely packed electrodes.
From an engineering perspective, one of the primary challenges with miniaturizing MEAs and decreasing pixel size is the corresponding increase in charge density [30]. With decreasing pixel electrode size, there is less surface area available to transfer charge to the surrounding tissue. However, retinal cells require a minimum amount of charge to meet the activation threshold needed in order to relay visual information [31]. Consequently, to provide a similar electric field penetration depth, a higher electrode charge density per surface area is necessary when using smaller electrodes [32]. Due to the limitations of material properties and safety requirements, it is, nevertheless, challenging to accommodate this increased charge-density requirement [33][34]. Conventional platinum electrodes, widely used for visual prostheses, exceed the charge injection limits for safe stimulation with decreased pixel size [30]. To overcome this challenge, innovations in the field of material science have proposed modifications to retinal prostheses’ MEAs. For instance, researchers manufactured nanocone-shaped platinum–iridium oxide neural microelectrodes to increase the electrode surface area [35]. Others incorporated high-conductivity materials such as graphene, materials with superior charge injection such as polymer/carbon nanotubes, and carbon nanotube-modified gold [30][36][37]. These innovations made it possible to have efficient charge injection and opened the horizon to decreasing the electrode size on MEAs, with a final objective of improving the resolution limit of retinal prostheses [38]. However, with each of the proposed materials, biocompatibility, manufacturability, and batch-to-batch consistency are still important considerations that need to be studied to maximize the benefits while ensuring that the risks and variability are minimized.
3.3. Spatial Selectivity
Electrical stimulation by MEAs allows surviving RGCs to depolarize and transmit visual signals to the brain [39]. The outcome achieved by these signals is a phosphene–the basic unit of artificial vision, defined as a subjective “visual percept” experienced by recipients of retinal prostheses. Unsurprisingly, in an ethnographic, qualitative account of how the current implementation of retinal prostheses translates into the perceptual experience of patients, the recipients of retinal prostheses described their vision as distinctly different from that of natural vision and as most closely resembling a “light show” [40]. These manifest differences between artificial and natural vision can be attributed to the coarse pattern of retinal activation caused by the MEA stimulation of many neighboring cells without coordination [39]. However, it is not necessarily the difference from natural vision that is concerning, rather, it is the unreliable and irregularly shaped phosphenes that are produced as a result of such coarse and unselective activation [41]. When an electric field is produced by a stimulating electrode, the shape of the electric field and its strength directly impact the visual percept of the recipient. If the electric field spreads in a lateral direction with distance above the electrode, it can simultaneously activate many retinal cells and cause a loss of spatial selectivity (Figure 8) [24].

Figure 8. (Left) Electric field of a bipolar configuration causing lateral spread and unselectively stimulating many retinal cells. (Right) A 3D geometry electrode with circumferential returns generates locally confined electric fields, reducing electrode cross-talk and permitting more selective activation of retinal cells. (Figure 8 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
In epiretinal devices, MEAs inadvertently activate a bundle of RGC axons in the nerve fiber layer. This bundle activation happens since the axons of RGCs lie closer to the electrodes than the underlying RGC soma that are the intended targets of stimulation [42]. The result of this activation is elongated or arc-like phosphenes that negatively impact the visual quality [43]. Similarly, while subretinal prosthesis are largely spared from the interference by RGC axons, the unwanted excitation from these electrodes can result in highly variable phosphene shapes across electrodes and between subjects [44]. This phenomenon happens due to the activation of many cells and cell types, including tertiary retinal neurons such as amacrine cells [45][46].
If the single electrodes of retinal prostheses can reliably produce an individual, isolated point of light, which the brain can then assemble into objects similar to an electronic scoreboard, the spatial resolution and consequent vision can be drastically improved [47]. However, this is not yet possible due to unselective and imprecise retinal stimulation. The engineering solutions to this challenge have varied, and two predominant methods have been proposed. Firstly, there is the use of return electrodes to localize the electric field and, thereby, selectively stimulate cells. Secondly, there is the manipulation of the electric stimulation parameters, such as the amplitude and frequency, which can selectively target cells that only respond to those specific parameters and not to others.
3.3.1. Return Electrodes for Electric Field Localization and Current Steering
The stimulation of retinal cells is mainly achieved by depolarizing cells in an electric field, instead of direct current injection into cells [24]. By accumulating charge at the synaptic terminals of retinal cells, the membrane depolarization exceeds a threshold, and action potentials can be fired. Confining the electric field and controlling its shape is important to ensure the stimulation selectivity of the desired target cells. To achieve such control, researchers manipulated MEAs to incorporate adjacent return or grounding electrodes. Return electrodes serve as the electrical counterpart to the active electrodes that are implanted in the retina, and, through them, it is possible to limit current spread and produce a well-controlled, directed electric field. A variety of return electrodes were widely investigated for use in retinal prostheses including monopolar, bipolar, tripolar, and hexapolar configurations. As seen in Figure 9, in the monopolar configuration, a single stimulating electrode is used to activate retinal cells. The bipolar configuration has an active electrode with an adjacent return electrode, which allows for current steering and localization. In the tripolar configuration, two electrodes at the opposite sides of the stimulating electrode function as the return electrodes. Finally, in the hexapolar configuration, an active electrode is centrally located and is surrounded by six return electrodes that form a “guard” around the active electrode [24]. In addition to these main designs, more complex configurations were recently explored and proposed for further testing. For instance, a 3D multilayered, concentric bipolar electrode was designed with the purpose of achieving highly focused electric stimulation [48]. Another research group developed a method that enables dynamic electric field confinement, by turning the designated active pixels into transient returns [45]. In addition to these designs, researchers also developed a computational implant simulator that can model electric fields of MEAs [26]. This simulator was able to compute the electric field in the retina generated by thousands of electrodes as well as with various pixel configuration [26]. It is through such computational models as well as through the unique ideation and implementation of return electrodes that it can be possible to confine the electric field and selectively target desired cells [39].
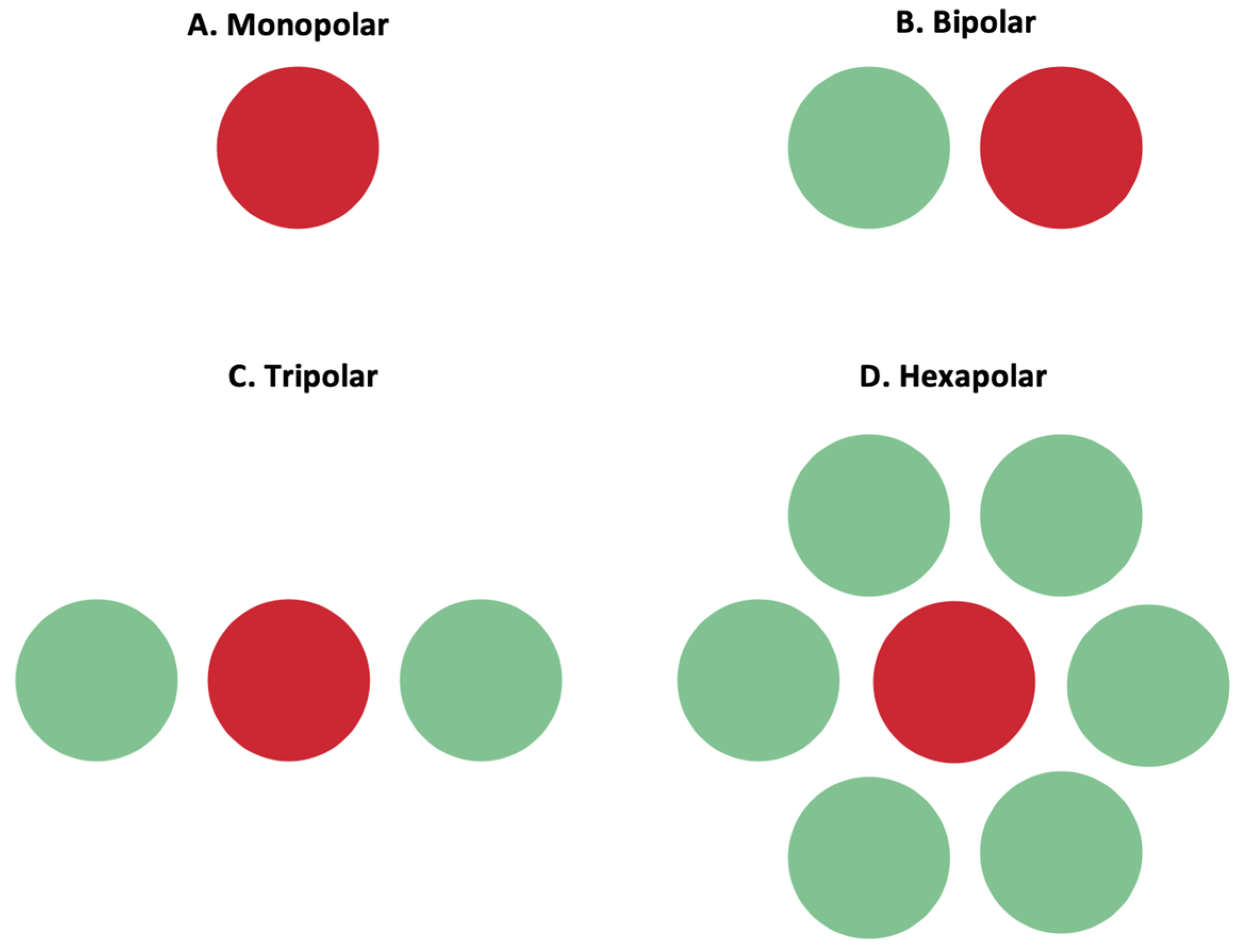
Figure 9. Active (red) and return (green) electrodes arranged in different configurations. (A) Monopolar configuration; (B) bipolar configuration; (C) tripolar configuration; (D) hexapolar configuration. These configurations impact the shape of the electric field, and the return electrode is used to limit current spread and produce a well-controlled, directed electric field.
However, while current steering is important to ensure that small-sized electrodes can target one cell at a time, it does not overcome the challenge of bundle activation in the nerve fiber layer. Especially in epiretinal electrodes, where the nerve fiber layer more proximally lies to the electrode than to the more distal RGC somas, another method of cell stimulation is needed to selectively activate the somas [24]. Control of the electric stimulation parameters was proposed as a solution.
3.3.2. Electric Stimulation Parameters for Selective Cell Activation and Chromatic Vision
An alternative approach to the selective activation of retinal cells is to manipulate the electric impulses sent by the electrodes [24]. That is, instead of manipulating the electric field shape and confinement to optimize selective cell activation, electrical stimulation strategies were proposed to optimally target specific cells and leave other cells inactivated. This stimulation strategy requires finding the pattern of electrical stimulation, including the amplitude and frequency of the electrical pulse, which permits targeted retinal activation. Then, these impulses are sent through the MEAs to activate the retinal cells. Two main reasons were suggested that favor this approach over electric field confinement. Firstly, researchers showed that there may be more than 40 types of RGCs, and each may be responsible for different aspects of visual information processing [49]. Selectively stimulating each of these types of RGCs would be desirable for the full restoration of vision [47]. Individualized electrical stimuli from the independent electrodes on MEAs can be used to target different types of cells, such as ON- and OFF- cells. Moreover, researchers found that the response to an electric pulse produced by RGCs is also dependent on the patient disease genotype [50]. Patients with varying genotypes of retinitis pigmentosa would, therefore, have varying quality of prosthetic vision, even though the same device may be implanted in all patients. Through their study, the authors asserted the importance of exploring novel stimulation strategies to enhance the response ratio between ON- and OFF- cell responses, such that these strategies can be customized for different genotypes. If only electric field confinement was used, it would not be possible to obtain the desirable output responses for the different cell types and different disease genotypes. The second reason for using electrical stimulation parameters in retinal prostheses is that researchers found that frequency-modulated electrical stimulation can provide hope for chromatic vision restoration in blind patients [51]. Stanga et al. (2011) first discovered that color perception could be evoked by changing the relevant stimulation parameters [52]. In their clinical trial, nine subjects with retinitis pigmentosa were equipped with the Argus II retinal prosthesis system [52]. The study methodology involved the simultaneous stimulation of various electrode pairs using different permutations of cathodic–anodic pulses [52]. The subjects reported eight different colors including orange, red, blue, green, and white; blue, yellow, and white were perceived the most [52]. A subsequent study in 2012 enrolled four blind subjects diagnosed with retinitis pigmentosa, who were equipped with the Argus II retinal prosthesis system [53]. The findings of this study demonstrated that it is possible to simultaneously evoke two distinct colored flashes, a significant difference from the first study [53]. In total, the patients perceived seven color combinations: gray–white, yellow–gray, orange–white, white–blue, brown–white, yellow–white, and yellow–blue [53]. The authors then concluded that manipulating the stimulation parameters can provide rudimentary color vision to blind patients and allow them to simultaneously perceive multiple colors, depending on stimulation parameters [52][53]. More recently, V L Towle et al. (2021) reached a similar conclusion and suggested that color hue development in prostheses may be dependent on stimulus intensity [54]. V L Towle and colleagues designed a study and were able to demonstrate an “amplitude-frequency” stimulation strategy to encode color vision, which was validated with experimental data [55]. Additionally, a case series study that followed seven subjects blinded by advanced RP and fitted with an epiretinal prosthesis showed that five/seven subjects perceived chromatic vision when frequency-modulated electrical stimulation of the retina was tested [51]. In all cases, color vision was achieved by selectively targeting small bistratified cells [51]. The knowledge that targeting specific cell types with specific parameters can restore some color percepts to patients creates an immense value for implementing the electric stimulation parameters that can selectively activate those cells. Moreover, unlike the electric field confinement method, the incorporation of electric stimulation can preferentially activate RGCs’ somas (versus passing axons in the nerve fiber layer) by manipulating the stimulus’ durations, phases, and waveforms [56].
While the two methods for selective stimulation are presented in comparison with each other, it should be noted that the use of one method does not exclude the use of the other. Indeed, both methods can and have been amalgamated to achieve higher selective activation. Activation through current steering avoids lateral stimulation and cross-talk, while using electric stimulation strategies can aid in targeting certain cells that are only responsive to that stimulus [29]. For instance, Jepson et al. (2014) investigated the usage of current steering in retinal prosthetics using multiple, densely packed suprachoroidal electrodes in the macaque retina, while providing simultaneous stimulation parameters for these electrodes [57]. Jepson and colleagues identified that when a current is applied to two adjacent electrodes, a peak current is produced at an equidistant point, termed a virtual electrode. Modulating the current ratio creates biased electric fields; in other words, if one electrode receives a higher current, the virtual electrode shifts toward that electrode. The authors predicted that by using current steering to optimize the stimulation intensity, frequency, and number of electrodes, it could be possible to increase target cell selectivity while reducing the response probability of non-target cells. Since it is challenging to document every stimulus pattern permutation, the authors proposed a piecewise linear model. They then measured the produced pattern and found that it was almost identical to the spatial pattern predicted by the model, “allowing for 0.90 [~90%] activation probability of the target cell with only 0.11 [~11%] probability of activating the neighboring cell [57]”.
3.4. Bidirectional/Closed-Loop Retinal Prostheses
One of the recent advances in the field of retinal prostheses is the design and development of bidirectional and closed-loop systems. These systems are able to both send electric impulses to retinal cells and record the electric responses evoked by stimulation [58]. The increased emphasis on this area in recent research can be attributed to several reasons. The first reason is the neural plasticity of the retina. With disease progression, photoreceptors within the retina can degrade. In response, the retina remodels, altering the electrophysiologic properties of the retinal pathways [59]. Due to this dynamic change in the electrophysiologic properties over the disease course and the consequent interpatient differences, the electrical patterns used to stimulate retinal cells need to be iteratively manipulated to produce an optimal outcome for patients [60]. For instance, as recently demonstrated by Caravaca-Rodriguez et al. (2022), the progressive degeneration of the retina resulted in an increased electrical threshold needed by subretinal and epiretinal prostheses [61]. The second reason driving innovation in the development of bidirectional systems is that these systems can enable fully representative, personalized, and iterative stimulation strategies. Often, to test stimulation parameters, including stimulus amplitude, width, and time, experiments are conducted in ex vivo conditions. These conditions, however, lack the metabolic environment of the eye, upon which the true electrophysiologic properties are dependent [58]. Thus, in vivo, bidirectional systems can provide a more complete reflection of the retinal response to stimulation and fill in any areas of uncertainty of ex vivo testing. Finally, bidirectional systems are important, since, as mentioned in previous sections, there is often a variable electrode–retina distance upon implantation [12]. This variability in electrode–retina distance impacts the stimulation ability and the produced phosphenes [44]. Thus, having closed-loop systems that guide the required stimulation of individual electrodes based on the evoked cell response can significantly improve patient outcomes. These closed-loop systems would enable such post-implantation customization.
In addition to these factors, closed-loop systems also help address some of the previously mentioned biological challenges. For instance, Tandon et al. (2021) implemented bidirectional epiretinal prostheses to develop an algorithm for detecting axon bundle activation [41]. Similarly, Ghaffari et al. (2021) developed a closed-loop neural network (NN) model of RGC spatial activity and used it to create a real-time optimization method to search for stimulation parameters that elicit focal responses from in vitro retina [62].
The incorporation of bidirectional closed-loop systems into retinal prostheses requires an understanding of how the recorded cell response can then be used to feedback stimulation strategies to target cells [39]. As such, there has been an influx of computational models that allow researchers and engineers to understand the electrical activity of the retina and the impact that different stimulation parameters have on different cell types at varying levels of degeneration [63][64][65][66][67].
This entry is adapted from the peer-reviewed paper 10.3390/s23135782
References
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Kolb, H.; Fernandez, E.; Nelson, R. Anatomy and Physiology of the Retina; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995.
- Abbasi, B.; Rizzo, J.F. Advances in Neuroscience, Not Devices, Will Determine the Effectiveness of Visual Prostheses. Semin. Ophthalmol. 2021, 36, 168–175.
- Mirochnik, R.M.; Pezaris, J.S. Contemporary Approaches to Visual Prostheses. Mil. Med. Res. 2019, 6, 19.
- Ghezzi, D. Translation of a Photovoltaic Retinal Prosthesis. Nat. Biomed. Eng. 2020, 4, 137–138.
- Cehajic-Kapetanovic, J.; Singh, M.S.; Zrenner, E.; MacLaren, R.E. Bioengineering Strategies for Restoring Vision. Nat. Biomed. Eng. 2022, 7, 387–404.
- Zrenner, E.; Bartz-Schmidt, K.U.; Benav, H.; Besch, D.; Bruckmann, A.; Gabel, V.-P.; Gekeler, F.; Greppmaier, U.; Harscher, A.; Kibbel, S.; et al. Subretinal Electronic Chips Allow Blind Patients to Read Letters and Combine Them to Words. Proc. Biol. Sci. 2011, 278, 1489–1497.
- Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L.; Kamins, T.I.; Galambos, L.; Smith, R.; Harris, J.S.; Sher, A.; et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nat. Photonics 2012, 6, 391–397.
- Huang, T.W.; Kamins, T.I.; Chen, Z.C.; Wang, B.-Y.; Bhuckory, M.; Galambos, L.; Ho, E.; Ling, T.; Afshar, S.; Shin, A.; et al. Vertical-Junction Photodiodes for Smaller Pixels in Retinal Prostheses. J. Neural Eng. 2021, 18, 036015.
- Tong, W.; Stamp, M.; Apollo, N.V.; Ganesan, K.; Meffin, H.; Prawer, S.; Garrett, D.J.; Ibbotson, M.R. Improved Visual Acuity Using a Retinal Implant and an Optimized Stimulation Strategy. J. Neural Eng. 2019, 17, 016018.
- Xu, Y.; Pang, S. Microelectrode Array With Integrated Pneumatic Channels for Dynamic Control of Electrode Position in Retinal Implants. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2292–2298.
- Avraham, D.; Yitzhaky, Y. Simulating the Perceptual Effects of Electrode–Retina Distance in Prosthetic Vision. J. Neural Eng. 2022, 19, 035001.
- Flores, T.; Huang, T.; Bhuckory, M.; Ho, E.; Chen, Z.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Honeycomb-Shaped Electro-Neural Interface Enables Cellular-Scale Pixels in Subretinal Prosthesis. Sci. Rep. 2019, 9, 10657.
- Seo, H.W.; Kim, N.; Ahn, J.; Cha, S.; Goo, Y.S.; Kim, S. A 3D Flexible Microelectrode Array for Subretinal Stimulation. J. Neural Eng. 2019, 16, 056016.
- Vu, Q.A.; Seo, H.W.; Choi, K.-E.; Kim, N.; Kang, Y.N.; Lee, J.; Park, S.-H.; Kim, J.T.; Kim, S.; Kim, S.-W. Structural Changes in the Retina after Implantation of Subretinal Three-Dimensional Implants in Mini Pigs. Front. Neurosci. 2022, 16, 1010445.
- Seo, H.W.; Kim, N.; Kim, S. Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement. Micromachines 2020, 11, 467.
- Flores, T.; Lei, X.; Huang, T.; Lorach, H.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Optimization of Pillar Electrodes in Subretinal Prosthesis for Enhanced Proximity to Target Neurons. J. Neural Eng. 2018, 15, 036011.
- Abbott, C.J.; Baglin, E.K.; Kolic, M.; McGuinness, M.B.; Titchener, S.A.; Young, K.A.; Yeoh, J.; Luu, C.D.; Ayton, L.N.; Petoe, M.A.; et al. Interobserver Agreement of Electrode to Retina Distance Measurements in a Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis. Transl. Vis. Sci. Technol. 2022, 11, 4.
- Sharafkhani, N.; Kouzani, A.Z.; Adams, S.D.; Long, J.M.; Lissorgues, G.; Rousseau, L.; Orwa, J.O. Neural Tissue-Microelectrode Interaction: Brain Micromotion, Electrical Impedance, and Flexible Microelectrode Insertion. J. Neurosci. Methods 2022, 365, 109388.
- Zhou, M.; Kang, D.H.; Kim, J.; Weiland, J.D. Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics. Micromachines 2020, 11, 392.
- Ferlauto, L.; Airaghi Leccardi, M.J.I.; Chenais, N.A.L.; Gilliéron, S.C.A.; Vagni, P.; Bevilacqua, M.; Wolfensberger, T.J.; Sivula, K.; Ghezzi, D. Design and Validation of a Foldable and Photovoltaic Wide-Field Epiretinal Prosthesis. Nat. Commun. 2018, 9, 992.
- Vagni, P.; Airaghi Leccardi, M.J.I.; Vila, C.-H.; Zollinger, E.G.; Sherafatipour, G.; Wolfensberger, T.J.; Ghezzi, D. POLYRETINA Restores Light Responses in Vivo in Blind Göttingen Minipigs. Nat. Commun. 2022, 13, 3678.
- Rincón Montes, V.; Gehlen, J.; Ingebrandt, S.; Mokwa, W.; Walter, P.; Müller, F.; Offenhäusser, A. Development and in Vitro Validation of Flexible Intraretinal Probes. Sci. Rep. 2020, 10, 19836.
- Tong, W.; Meffin, H.; Garrett, D.J.; Ibbotson, M.R. Stimulation Strategies for Improving the Resolution of Retinal Prostheses. Front. Neurosci. 2020, 14, 262.
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Sahel, J.A. Simultaneous Perception of Prosthetic and Natural Vision in AMD Patients. Nat. Commun. 2022, 13, 513.
- Chen, Z.C.; Wang, B.-Y.; Goldstein, A.K.; Butt, E.; Mathieson, K.; Palanker, D. Photovoltaic Implant Simulator Reveals Resolution Limits in Subretinal Prosthesis. J. Neural Eng. 2022, 19, 055008.
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Muqit, M.; Sahel, J.A. Photovoltaic Restoration of Central Vision in Atrophic Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1097–1104.
- Liu, Z.; Kurokawa, K.; Zhang, F.; Lee, J.J.; Miller, D.T. Imaging and Quantifying Ganglion Cells and Other Transparent Neurons in the Living Human Retina. Proc. Natl. Acad. Sci. USA 2017, 114, 12803–12808.
- Shim, S.; Eom, K.; Jeong, J.; Kim, S.J. Retinal Prosthetic Approaches to Enhance Visual Perception for Blind Patients. Micromachines 2020, 11, 535.
- Zheng, X.S.; Yang, Q.; Vazquez, A.L.; Tracy Cui, X. Imaging the Efficiency of Poly(3,4-Ethylenedioxythiophene) Doped with Acid-Functionalized Carbon Nanotube and Iridium Oxide Electrode Coatings for Microstimulation. Adv. NanoBiomed Res. 2021, 1, 2000092.
- Xu, Z.; Lu, Y.; Qin, S.; Wu, T.; Qin, B. Electrical Stimulation Scheme Optimization for Retinal Prosthesis: Considerations from Biological Perspective. Ann. Eye Sci. 2020, 5, 13.
- Weiland, J.D.; Fink, W.; Humayun, M.; Liu, W.; Rodger, D.C.; Tai, Y.-C.; Tarbell, M. Progress Towards A High-Resolution Retinal Prosthesis. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2005; pp. 7373–7375.
- Cui, H.; Xie, X.; Xu, S.; Chan, L.L.H.; Hu, Y. Electrochemical Characteristics of Microelectrode Designed for Electrical Stimulation. BioMed. Eng. OnLine 2019, 18, 86.
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Kochnev Goldstein, A.; Palanker, D. Pixel Size Limit of the PRIMA Implants: From Humans to Rodents and Back. J. Neural Eng. 2022, 19, 055003.
- Zeng, Q.; Yu, S.; Fan, Z.; Huang, Y.; Song, B.; Zhou, T. Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials 2022, 12, 3445.
- Nguyen, D.; Valet, M.; Dégardin, J.; Boucherit, L.; Illa, X.; de la Cruz, J.; Del Corro, E.; Bousquet, J.; Garrido, J.A.; Hébert, C.; et al. Novel Graphene Electrode for Retinal Implants: An in Vivo Biocompatibility Study. Front. Neurosci. 2021, 15, 615256.
- Vafaiee, M.; Mohammadpour, R.; Vossoughi, M.; Asadian, E.; Janahmadi, M.; Sasanpour, P. Carbon Nanotube Modified Microelectrode Array for Neural Interface. Front. Bioeng. Biotechnol. 2020, 8, 582713.
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Yiliguma; Wang, Z.; Xue, T.; Jiang, M.; Zhang, J.; Zheng, G. Nanowire Arrays Restore Vision in Blind Mice. Nat. Commun. 2018, 9, 786.
- Fan, V.H.; Grosberg, L.E.; Madugula, S.S.; Hottowy, P.; Dabrowski, W.; Sher, A.; Litke, A.M.; Chichilnisky, E.J. Epiretinal Stimulation with Local Returns Enhances Selectivity at Cellular Resolution. J. Neural Eng. 2019, 16, 025001.
- Erickson-Davis, C.; Korzybska, H. What Do Blind People “See” with Retinal Prostheses? Observations and Qualitative Reports of Epiretinal Implant Users. PLoS ONE 2021, 16, e0229189.
- Tandon, P.; Bhaskhar, N.; Shah, N.; Madugula, S.; Grosberg, L.; Fan, V.H.; Hottowy, P.; Sher, A.; Litke, A.M.; Chichilnisky, E.J.; et al. Automatic Identification of Axon Bundle Activation for Epiretinal Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2496–2502.
- Grosberg, L.E.; Ganesan, K.; Goetz, G.A.; Madugula, S.S.; Bhaskhar, N.; Fan, V.; Li, P.; Hottowy, P.; Dabrowski, W.; Sher, A.; et al. Activation of Ganglion Cells and Axon Bundles Using Epiretinal Electrical Stimulation. J. Neurophysiol. 2017, 118, 1457–1471.
- Weitz, A.C.; Nanduri, D.; Behrend, M.R.; Gonzalez-Calle, A.; Greenberg, R.J.; Humayun, M.S.; Chow, R.H.; Weiland, J.D. Improving the Spatial Resolution of Epiretinal Implants by Increasing Stimulus Pulse Duration. Sci. Transl. Med. 2015, 7, 318ra203.
- Ghaffari, D.H.; Chang, Y.-C.; Mirzakhalili, E.; Weiland, J.D. Closed-Loop Optimization of Retinal Ganglion Cell Responses to Epiretinal Stimulation: A Computational Study. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual Event, Italy, 4–6 May 2021; pp. 597–600.
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Huang, T.; Shin, A.; Zuckerman, V.; Ho, E.; Rosenfeld, E.; Galambos, L.; Kamins, T.; et al. Electronic Photoreceptors Enable Prosthetic Visual Acuity Matching the Natural Resolution in Rats. Nat. Commun. 2022, 13, 6627.
- Madugula, S.S.; Gogliettino, A.R.; Zaidi, M.; Aggarwal, G.; Kling, A.; Shah, N.P.; Vilkhu, R.; Hays, M.R.; Nguyen, H.; Fan, V.; et al. Focal Electrical Stimulation of Human Retinal Ganglion Cells for Vision Restoration. J. Neural Eng. 2022, 19, 066040.
- Yunzab, M.; Soto-Breceda, A.; Maturana, M.; Kirkby, S.; Slattery, M.; Newgreen, A.; Meffin, H.; Kameneva, T.; Burkitt, A.N.; Ibbotson, M.; et al. Preferential Modulation of Individual Retinal Ganglion Cells by Electrical Stimulation. J. Neural Eng. 2022, 19, 045003.
- Borda, E.; Gaillet, V.; Airaghi Leccardi, M.J.I.; Zollinger, E.G.; Moreira, R.C.; Ghezzi, D. Three-Dimensional Multilayer Concentric Bipolar Electrodes Restrict Spatial Activation in Optic Nerve Stimulation. J. Neural Eng. 2022, 19, 036016.
- Goetz, J.; Jessen, Z.F.; Jacobi, A.; Mani, A.; Cooler, S.; Greer, D.; Kadri, S.; Segal, J.; Shekhar, K.; Sanes, J.R.; et al. Unified Classification of Mouse Retinal Ganglion Cells Using Function, Morphology, and Gene Expression. Cell Rep. 2022, 40, 111040.
- Roh, H.; Otgondemberel, Y.; Eom, J.; Kim, D.; Im, M. Electrically-Evoked Responses for Retinal Prostheses Are Differentially Altered Depending on Ganglion Cell Types in Outer Retinal Neurodegeneration Caused by Crb1 Gene Mutation. Front. Cell. Neurosci. 2023, 17, 1115703.
- Yue, L.; Castillo, J.; Gonzalez, A.C.; Neitz, J.; Humayun, M.S. Restoring Color Perception to the Blind: An Electrical Stimulation Strategy of Retina in Patients with End-Stage Retinitis Pigmentosa. Ophthalmology 2021, 128, 453–462.
- Stanga, P.E.; Hafezi, F.; Sahel, J.A.; daCruz, L.; Merlini, F.; Coley, B.; Greenberg, R.J.; Argus II Study Group. Patients Blinded By Outer Retinal Dystrophies Are Able To Perceive Color Using The ArgusTm II Retinal Prosthesis System. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4949.
- Stanga, P.E.; Sahel, J.A., Jr.; daCruz, L.; Hafezi, F.; Merlini, F.; Coley, B.; Greenberg, R.J.; Argus II Study Group. Patients Blinded by Outer Retinal Dystrophies Are Able to Perceive Simultaneous Colors Using the Argus® II Retinal Prosthesis System. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6952.
- Towle, V.L.; Pham, T.; McCaffrey, M.; Allen, D.; Troyk, P.R. Toward the Development of a Color Visual Prosthesis. J. Neural Eng. 2021, 18, 023001.
- Paknahad, J.; Loizos, K.; Yue, L.; Humayun, M.S.; Lazzi, G. Color and Cellular Selectivity of Retinal Ganglion Cell Subtypes through Frequency Modulation of Electrical Stimulation. Sci. Rep. 2021, 11, 5177.
- Chang, Y.-C.; Ghaffari, D.H.; Chow, R.H.; Weiland, J.D. Stimulation Strategies for Selective Activation of Retinal Ganglion Cell Soma and Threshold Reduction. J. Neural Eng. 2019, 16, 026017.
- Jepson, L.H.; Hottowy, P.; Mathieson, K.; Gunning, D.E.; Dąbrowski, W.; Litke, A.M.; Chichilnisky, E.J. Spatially Patterned Electrical Stimulation to Enhance Resolution of Retinal Prostheses. J. Neurosci. 2014, 34, 4871–4881.
- Vėbraitė, I.; Hanein, Y. In the Eye of the Storm: Bi-Directional Electrophysiological Investigation of the Intact Retina. Front. Neurosci. 2022, 16, 829323.
- Caravaca-Rodriguez, D.; Gaytan, S.P.; Suaning, G.J.; Barriga-Rivera, A. Implications of Neural Plasticity in Retinal Prosthesis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11.
- Kang, H.; Abbasi, W.H.; Kim, S.-W.; Kim, J. Fully Integrated Light-Sensing Stimulator Design for Subretinal Implants. Sensors 2019, 19, 536.
- Xu, A.; Beyeler, M. Retinal Ganglion Cells Undergo Cell Type-specific Functional Changes in a Biophysically Detailed Model of Retinal Degeneration. BioRxiv 2023.
- Haji Ghaffari, D.; Akwaboah, A.D.; Mirzakhalili, E.; Weiland, J.D. Real-Time Optimization of Retinal Ganglion Cell Spatial Activity in Response to Epiretinal Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2733–2741.
- Ly, K.; Guo, T.; Tsai, D.; Muralidharan, M.; Shivdasani, M.N.; Lovell, N.H.; Dokos, S. Simulating the Impact of Photoreceptor Loss and Inner Retinal Network Changes on Electrical Activity of the Retina. J. Neural Eng. 2022, 19, 065002.
- Paknahad, J.; Kosta, P.; Bouteiller, J.-M.C.; Humayun, M.S.; Lazzi, G. Mechanisms Underlying Activation of Retinal Bipolar Cells through Targeted Electrical Stimulation: A Computational Study. J. Neural Eng. 2021, 18, 066034.
- Iseri, E.; Kosta, P.; Paknahad, J.; Bouteiller, J.-M.C.; Lazzi, G. A Computational Model Simulates Light-Evoked Responses in the Retinal Cone Pathway. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 4482–4486.
- Italiano, M.L.; Guo, T.; Lovell, N.H.; Tsai, D. Improving the Spatial Resolution of Artificial Vision Using Midget Retinal Ganglion Cell Populations Modeled at the Human Fovea. J. Neural Eng. 2022, 19, 035002.
- Relic, L.; Zhang, B.; Tuan, Y.-L.; Beyeler, M. Deep Learning-Based Perceptual Stimulus Encoder for Bionic Vision. In Proceedings of the Augmented Humans International Conference, Chiba, Japan, 13–15 March 2022.
This entry is offline, you can click here to edit this entry!
