Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Nanomedicine presents innovative solutions for cancer treatment, including photothermal therapy (PTT). PTT centers on the design of photoactivatable nanoparticles capable of absorbing non-toxic near-infrared light, generating heat within target cells to induce cell death. The successful transition from benchside to bedside application of PTT critically depends on the core properties of nanoparticles responsible for converting light into heat and the surface properties for precise cell-specific targeting. Precisely targeting the intended cells remains a primary challenge in PTT.
- photothermal therapy
- biomimetic nanoparticles
- membrane-camouflaged nanoparticles
- cancer treatment
1. Introduction
1.1. Photothermal Therapy for Cancer Treatment
Cancer is still one of the most significant challenges of the twenty-first century [1]. The complexity of the condition (many diseases encompassed in one name) and the high heterogeneity of individual tumors themselves contribute to the difficulty of this challenge [2]. Immunotherapy has recently joined the three classic pillars of clinical cancer treatment (surgery, chemotherapy, and radiotherapy), bringing new hope to oncological patients [3]. Moreover, many other treatment modalities are getting increasingly closer to clinical application. This selection of different therapeutic modalities is needed due to cancer’s complexity. Within these new modalities, one that has already reached the clinic in some form is hyperthermia [4].
Heating up cancer tissues can treat the disease due to the increased susceptibility of tumor cells to temperature. On top of that, a T increase in the tumor microenvironment (TME) can facilitate the penetration of drugs and medical formulations deep into solid tumors. Photothermal therapy (PTT) is one of the most promising versions of hyperthermia due to its safety and high externally controlled specificity. In PTT, a material (photothermal agent, PTA) acts as an intermediary between a radiation source and the desired toxic effect on cancer cells by converting electromagnetic radiation into heat. In terms of the excitation source, near-infrared (NIR) wavelength is preferred, mainly for three reasons: (i) longer wavelength radiation can penetrate deeper into biological tissues; (ii) NIR falls within what is known as biological windows (wavelengths where the absorption by biomolecules is reduced); and (iii) NIR radiation energy is not high enough to induce biochemical changes on healthy cells and thus is safer than, e.g., UV radiation (able to damage DNA) [5].
The high specificity and safety of PTT rely on the two-component nature of the treatment: radiation source and photothermal agent. The exposure of a tissue to (NIR) laser radiation is not enough to generate a temperature change on its own. In the same way, the presence of photothermal agents should not produce any toxic effect on its own. This two-component nature is of the utmost importance as it allows us to surpass some limitations of irradiation systems and nanoparticle-based drugs. On the side of the irradiation, as the electromagnetic radiation passes through healthy tissues on its way to the tumor, it will not exert any deleterious effect on those tissues as long as no PTA is present. Modern laser systems provide high control over the irradiated area and stability of the radiation, spectral width, and power output. On the PTA side, many of the most successful PTAs are nanoparticle-based (see below), and as such, they tend to accumulate in ‘filtering’ organs like the liver, the spleen, or the lungs. These organs will not suffer any deleterious effect from the PTAs as long as they are not exposed to the laser.
Inorganic materials tend to be cleared from circulation by the mononuclear phagocyte system, while organic molecules are rapidly excreted via the kidneys and frequently present solubility issues. Targeted strategies based on functionalizing these materials with specific antibodies, peptides, or aptamers have only improved tumor accumulation modestly [6]. In this scenario, biomimetic strategies using cell membranes and natural structures to coat PTAs are being heavily explored to improve circulation time and tumor accumulation.
1.2. Nanocarriers for Photothermal Therapy
As mentioned above, PTT relies on localized electromagnetic energy conversion into heat. Speaking in general, the outcome of the PTT process from the side of the photothermal agent depends on parameters inherent to the photothermal agent’s nature (mainly the photothermal conversion efficiency) plus effects that are more dependent on physicochemical parameters and/or related to the functionalization of these materials (e.g., specificity, cell penetration abilities) [7]. Photothermal conversion efficiency (PCE) has been extensively studied on Au nanostructures. For these particular plasmonic materials, the PCE depends mainly on the plasmon resonance wavelength, particle volume/size (PCE of Au nanoshells decreases with increasing size) [8], shell coating, and assembly [9]. For semiconductor materials, the band gap also plays a key role. Optimizing these materials is a lengthy process in which a compromise must be reached between PCE, synthesis complexity, functionalization, and biocompatibility/safety.
Since the introduction of nanotechnology into biomedicine, particularly oncology, the number of therapeutic options reported at a research level has increased significantly. This is also true in the area of PTT. There is currently a comprehensive battery of material options described as PTT agents. Among them, Au nanomaterials and polydopamine (PDA) nanostructures are arguably the most important. Nevertheless, others have shown excellent properties as PTT agents beyond these two materials. For example, carbon-based materials, either carbon nanotubes (CNTs) or graphene, have been widely studied in this field. Recently, semiconductor materials such as CuS, MoS2, Bi2S3, Bi2Se3, and black phosphorus have been introduced.
Once NPs are administered to a patient, biomolecules in the blood serum spontaneously form adsorption layers around the NPs called protein corona, which leads to recognition and faster clearance by the immune system. As a result, nanoparticle-targeting capabilities are profoundly reduced.
Coating the NPs’ core with an outer shell of different materials has evolved as an excellent strategy to obtain nanoparticles that can escape or attenuate the immune response. Among different approaches to shell fabrication, functionalization with biomolecules has been demonstrated to increase NPs’ stability in biological fluids and improve specific cancer targeting. The use of simple molecules, such as PEG, to modify the surface of the particles has been shown to reduce aggregation and lower protein corona formation. However, PEG can induce a specific immune response, producing anti-PEG antibodies that facilitate PEGylated nanoparticle phagocytosis.
Since traditional shells show significant limitations, innovative approaches based on the generation of biomimetic nanoparticles have emerged in the last few years. Biomimetic nanoparticles combine the desirable biocompatibility properties and immune evasion of natural entities (i.e., cell membranes, extracellular vesicles, toxins, and pathogens) and the tunable capacity of synthetic nanoparticles (Figure 1).
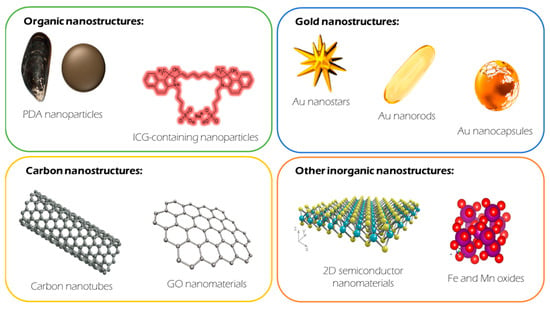
Figure 1. Main families of materials described as photothermal therapy agents and the most representative members within each family.
2. Core: NIR-Responsive Materials
2.1. Organic Materials
Two of the most commonly used materials for PTT fall within this category, polydopamine (PDA) and indocyanine green (ICG), together with other less-known materials such as polypyrrole or porphyrins (better known for their photodynamic properties). While PDA is used in the nanoparticle form (either as the primary or sole component of the nanostructure or as a coating on a nanoparticle of different nature) [10,11], ICG is frequently used as cargo within a nanostructured drug delivery system [12,13]. The main advantage of ICG is that it is already approved for human use for the diagnosis of cardiac, circulatory, hepatic, and ophthalmic conditions (“Definition of indocyanine green solution—NCI Drug Dictionary—NCI”, 2011) [14]; as such, a potential translation from bench to bedside is expected to be easier than other options. A covalent functionalization strategy would be preferred as it provides closer control over the fate of the dye. However, direct coupling of native ICG is chemically challenging, and thus, encapsulation/entrapment is the preferred option. ICG derivatives have been developed to solve this reactivity issue (e.g., IR808, IR825, IR2), but these structures lose the ease of translation advantage.
Regarding PDA, a bottom-up approach is usually followed for its preparation. Although several synthetic methods are available (enzymatic, electropolymerization) [11,15], the in situ oxidation of dopamine (DA) in solution is the most commonly used technique due to its simplicity. The mechanism of this polymerization is still controversial, although two main pathways seem to co-exist: oxidative polymerization and physical self-assembly [16]. This reaction is used for the production of PDA NPs to add a PDA coating on top of NPs of a different nature (the ability of DA to adhere to a plethora of different materials through either the amine or the catechol moiety aids the versatility of this approach) and for the production of PDA nanocapsules (usually by coating an intermediate NP as a template that is later selectively digested).
2.2. Gold (Au) Nanomaterials
Au nanomaterials are the best-studied inorganic nanomaterials for PTT applications. Relatively large nanostructures are needed for efficient PTT (nanorods, nanocapsules, nanostars) to increase NIR absorption by matching the laser wavelength and the Au nanomaterial surface plasmon. The absorption wavelength of the surface plasmon depends on different parameters depending on the structure of the Au nanomaterial. For example, in the case of nanorods, the aspect ratio is the parameter that controls the plasmon resonance. In the case of nanocapsules, the plasmon depends on the size and thickness of the Au shell, while in the case of nanostars, the plasmon depends on the size and thickness of the tip of these anisotropic materials [18]. The size/shape also controls the PCE of Au nanomaterials [19]. Smaller diameters generally lead to a higher PCE, while anisotropic shapes increase the PCE by increasing the absorption cross section [20].
2.3. Carbon-Based Nanomaterials
2.3.1. Carbon Nanotubes (CNTs)
CNTs are one of the most iconic structures of nanotechnology. These structures have been extensively studied and investigated for various applications, including biomedicine. PTT can be a target application for CNTs due to their strong light absorption capability in a wide wavelength range, including NIR and high PCE. CNTs are cylindrical graphite sp2 sheets that can be classified into single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs), depending on the number of concentric graphite layers. The optical properties of these materials depend on their diameter and chirality (relative orientation of the carbon hexagons concerning the tube axis); this last property is difficult to control with most synthetic protocols for SWCNTs, resulting in absorption spectra for these samples with several absorption peaks superimposed [23]. MWCNTs present simpler absorption spectra where the absorption is inversely proportional to the wavelength. Despite these absorption differences, both nanostructures have been widely investigated as PTT agents [24,25].
2.3.2. Graphene
Closely related to CNTs, graphene and graphene family (graphene oxide and reduced graphene oxide) nanomaterials are also being heavily explored as PTT agents. These materials also present a sp2 carbon structure. Graphene oxide (GO) is probably the most explored member of this family. GO is commonly prepared through chemical oxidation (e.g., Hummer’s method and modifications) [26] and the exfoliation of graphite. GO is also the precursor for preparing reduced GO (rGO) through the exposure of GO to reducing agents at relatively high temperatures. In rGO, the graphitic lattice is more conserved than in GO, which enhances the NIR absorption of the material [27]. However, as with CNTs, both GO and rGO have been proposed for PTT applications [28].
2.4. Other (Inorganic) Nanomaterials
2.4.1. Semiconductor Materials
Many of the members of this family are 2D materials (black P, MoS2, WS2, Bi2X3, WO3), similar in structure to graphene. As in the case of graphene, their preparation is possible through a top-down approach via chemical exfoliation. However, this protocol is usually time consuming, and the physicochemical properties of the final nanomaterials are, in most cases, difficult to control. Thus, bottom-up protocols have been developed to prepare most of these materials [29].
2.4.2. Oxide Materials
Fe and Mn oxides are versatile materials with a high multifunctional potential in the biomedical arena. Depending on their structure (many oxides are described for Fe and Mn with very different properties; γ-Fe2O3, α-Fe2O3, Fe3O4, MnO, MnO2, Mn3O4), Fe and Mn oxides can be used as contrast agents in magnetic resonance imaging (MRI) [35,36], as magnetic hyperthermia effectors [37], for magnetic guiding purposes [38], as cytotoxic probes through Fenton and Fenton-like reactions [39], and as O2 generating structures [40]; in addition, as mentioned above, Fe oxide nanoparticles (magnetite and maghemite) reached the clinic in the 1990s as MRI contrast agents and are still used worldwide as iron supplements.
3. Biomimetic Shells
3.1. Membrane-Camouflaged Nanoparticles
Recently, cell-derived membranes have been used to cover nanoparticles with external decoration to escape immune recognition and improve their blood circulation time. This process consists of the complete transfer of the native markers present on cell membranes or extracellular vesicles (EVs) of source cells onto the surface of the different nanomaterials. Different cell sources can be used to cloak nanoparticles using different coating approaches. This section will only focus on eukaryote-derived membranes.
3.1.1. Cell-Membrane-Cloaked Nanoparticles
Cell-membrane-camouflaged NPs are a top-down approach to coat synthetic NP cores with natural cellular membranes to avoid immune clearance and increase circulation time in the body, specificity, and efficacy. Researchers have camouflaged NPs with cell membranes from healthy cells (i.e., red blood cells, platelets, macrophages, MSC, fibroblasts) and cancer cells. Both cell sources have demonstrated their unique ability to increase the immune system’s shielding effect of protein corona and reduce phagocytosis.
Healthy Cell Membranes
The immune system can recognize “markers of self” on the surface of healthy cells, such as the CD47 protein on Erythrocytes (red blood cells, RBCs), which can bypass the clearance by macrophage engulfment. Effectively escaping immune recognition in the body also benefits prolonged blood circulation. Thus, researchers have taken advantage of the effective immune recognition in healthy cells to use them as natural carriers
Red blood cells—The application of healthy cells as vehicles of bioactive molecules was initially studied with RBCs. In 1953, Gardos reported the first encapsulation of a molecule into RBCs. However, Jain’s “carrier red blood cells” concept was not established until 1979 to describe drug-loaded erythrocytes [60].
Gold was the first photothermal conversion material to be cloaked with a cellular membrane, specifically, RBC membranes [62]. Although this study showed how solid gold nanospheres could be coated entirely with RBC vesicles to evade macrophage uptake, researchers did not further analyze the photothermal effect of AuNP@RBC on cancer cells. However, this first report laid the foundation for subsequent coating of NIR-responsive nanomaterials with RBC membranes for PTT, opening a new area of anti-cancer strategies [43,44]. Thus, Piao et al. reported the fabrication of RBC-membrane-coated gold nanocages (RBC-AuNCs) for studies of PTT [44]. They demonstrated the tumor uptake of NPs and cancer cell ablation via photothermal effects in vitro and in mice.
Besides gold, iron-oxide-based nanomaterials have been reported recently and are raising great interest for tumor PTT. For example, iron oxide magnetic nanoclusters (MNC) coated with RBC membranes (MNC@RBCs) demonstrated prolonged blood circulation time and significantly enhanced PTT efficacy. The treatment with MNC@RBCs and NIR light significantly reduced the average tumor weight using a breast cancer xenograft mouse model [45]. To increase the photothermal conversion efficacy of MNC@RBCs, Wang and co-workers loaded NIR cypate molecules to the MNC (Cyp-MNC@RBCs) [46]. The treatment with cyp-MNC@RBCs plus laser irradiation, compared to their MNC@RBCs counterpart, significantly inhibited tumor growth in HCT-116 tumor-bearing mice.
Current efforts to further improve NP biodistribution and tumor homing include using different NIR-responsive nanomaterials beyond gold and iron oxide and functionalizing RBC membranes with active targeting ligands. In addition, PTT is often combined with chemotherapy, and NPs are co-loaded with chemotherapeutic drugs to enhance the anti-cancer effect [47,48,49,50].
Platelets—Platelet (PLT)-membrane-camouflaged nanoparticles have also been widely explored for cancer therapy. It has been shown that coating with platelet membrane increases evasion of immune clearance while favoring interactions with damaged vasculature and tumor tissues [63]. For example, mesoporous silica-coated bismuth nanorods camouflaged by platelet membrane (BMSNR@PM) escaped the immune system by lowering macrophage endocytosis. Also, treatment with BMSNR@PM significantly lowered the tumor size of 4T1-tumor-bearing [51].
As occurs for RBM-camouflaged NPs, it is frequent to combine PTT with chemotherapy. Due to its efficacy in fighting many cancers, doxorubicin (DOX) is the most common anti-cancer drug used as a proof of concept. Wu et al. fabricated polypyrrole (PPy) nanoparticles (as photothermal agents) encapsulating DOX that were cloaked with PLT membranes [52]. The photo-chemotherapy based on PLT-PPy–DOX nanoparticles effectively suppresses primary tumor growth and inhibits tumor-distant-metastasis in an orthotopic mouse model of hepatocellular carcinoma. Another DOX example is PLGA-loaded nanoparticles combined with the NIR fluorescent dye IR780 for chemotherapy and PTT [53]. The IR780@PLGA/Dox nanoparticles camouflaged with PLT membranes showed longer retention times in the bloodstream of breast cancer 4T1-tumor-bearing Balb/c mice, which also accumulated at the tumor site.
Ferroptosis is a new type of programmed cell death discovered in 2012 that is mediated by iron-dependent Fenton-like reactions [65]. Iron accumulation leads to the continuous induction of lipid peroxidation, considered the leading killer in ferroptosis. Besides their use for PTT, iron oxide NPs can induce ferroptosis and are emerging as promising candidates for combinational anti-cancer therapy.
Macrophages—The Macrophage (Ma) membrane is also ideal for camouflaging NPs. Numerous studies have taken advantage of such biomimetic derivatives to increase biocompatibility, blood circulation, tumor accumulation, and decrease opsonization [55].
Amongst NIR-responsive materials, iron oxide and gold have been the preferred option to be camouflaged with macrophage membranes. An Fe3O4 core plus the macrophage membrane shell (Fe3O4@Ma) exhibit good biocompatibility, immune evasion, cancer targeting, and light-to-heat conversion capabilities [55]. In addition, Fe3O4@Ma NPs were shown to have a therapeutic effect in in vivo studies. BALB/c mice bearing MCF-7 tumor xenografts treated with Fe3O4@Ma NPs exhibited dramatic tumor regression over time [55]. Gold-nanoshell-coated mesoporous silica nanoparticles can also be cloaked with macrophage membranes, effectively reducing tumor growth on 4T1-tumor-bearing mice upon NIR irradiation [56].
Mesenchymal stem cells—Similar to TAMs, mesenchymal stem cells (MSC) have demonstrated migration towards tumor microenvironments and immunogenicity. Only a few works have been reported using MSC membranes for “camouflaging” NPs, and most of them used polydopamine (PDA) as the core. PDA is a bioinspired material with exciting features, simple preparation protocols and functionalization procedures, biocompatibility, free radical scavenging, and photothermal/photoacoustic properties [66].
Cancer Cell Membranes
Although technological success has been achieved in NP functionalization, one of the main obstacles is still the precise cancer homing. Cancer cell membranes are becoming a promising and efficient approach to overcome that issue. Cancer cells can recognize each other and adhere to one another through a mechanism known as homotypic binding [67].
Currently, strategies for developing effective cancer membrane-coated NPs are the same as those using healthy cell membranes. Researchers usually choose a NIR-responsive material as a core (mainly iron oxide, gold, CuS, or PDA) or mesoporous silica with a PTA with NIR fluorescence, such as ICG. PLGA has also been exploited in many NPs as the core in combination with ICG. Then, depending on the type of cancer to target, the selection of the outer shell will differ [68,69,70,71,72,73,74,75].
Li and co-workers published another interesting example. They designed a PLGA NP encapsulating perfluorocarbons (PFCs) with ICG and coated with the human lung A549 cancer cell membrane (AM-PP@ICGNPs) [69]. The cancer cell membrane modification could effectively enhance the circulation times of NPs by 1.7 times compared to NPs without membrane coating and 3 times to free ICG molecules. Also, AM-PP@ICGNPs showed excellent photothermal conversion efficiency, and with the homologous targeting ability, AM-PP@ICGNPs accumulated mainly in the tumor site in A549 xenografted tumor mice. Importantly, tumor growth inhibition was achieved in AM-PP@ICGNP-treated mice after mild localized laser irradiation (765 nm, 400 mW/cm2, 15 min), which confirms the great potential of photothermal therapy.
DCT is a DNA methylation inhibitor used to activate the gasdermin E (GSDME) gene, which is frequently methylated and silenced in most tumor cells. When the GSDME gene is demethylated and the protein can be expressed, this can be cleaved by activated caspase-3, which leads to a type of programmed cell death known as pyroptosis accompanied by inflammatory and immune responses [76]. The 4T1-PLGA@ICG/DCT nanoparticle combines chemical and photo treatments to synergistically induce cell pyroptosis so that NIR laser irradiation produces at the same time local hyperthermia, caspase-3 activation and DCT release.
Hybrid Cell Membranes
An important strategy that has emerged in the last few years is using hybrid cell membranes. The advantage of this approach overcoating with a single cell type membrane is the presence of specific proteins of both cell types, which should combine the functionalities from both cell membranes. For instance, the macrophage membrane presence helps NP avoid opsonization, while cancer cell membranes favor homotypic targeting. Therefore, combining both should improve NPs’ precise cancer homing and reduce immune clearance. In one example, photo-chemotherapy based on macrophage (RAW 264.7 cells)–cancer cell (H22) hybrid membrane-coated hollow copper sulfide NPs encapsulating sorafenib and surface modified with anti-VEGFR (CuS-SF@CMV NPs) was developed [73]. Sorafenib is a multi-kinase inhibitor that targets both MEK and VEGFR, and its effects were further enhanced by the inclusion of the anti-VEGFR antibody on the surface of CuS-SF@CMV NPs. The ~10 nm thick RAW 264.7-H22 uniform hybrid membrane contained specific membrane proteins of both cell types, such as the macrophage-specific CD135 (Fms-like tyrosine kinase 3) and the E-cadherin from the H22 cell. The authors reported that the presence of the hybrid membrane significantly increased the ability of the CuS@CM NPs to localize to the homotypic cells and escape the immune cells. Also, the in vivo murine hepatoma model treated with CuS@CM NPs and NIR irradiation exhibited smaller tumors than the control counterparts, confirming this approach’s potential therapeutic effect.
Bacterial-cancer cell-membrane-coated nanoparticles are another compelling example of using hybrid cell membranes. One advantage of using bacterial membranes is that the bacterial outer membrane presents immune activation properties. The outer membrane can trigger the production of anti-tumor cytokines by immune cells and induce the subsequent immune response for immunotherapy.
3.1.2. Cell-Derived Extracellular Vesicles
Recently, the use of extracellular vesicles (EVs), such as exosomes, as the natural material of biomimetic NPs is becoming of great interest. EVs are small membrane vesicles secreted by all types of cells that have an essential role in cell–cell communication. To reach the target sites and be internalized by the recipient cell, EVs present ligands in their surface membrane that bind to specific receptors in the host cells. The nature of ligands depends on the cells from which they originated [77,78]. Due to these surface ligands, EVs display a high targeting specificity and efficient cellular uptake. Considering EVs’ natural targeting capabilities and low immunogenicity, they are emerging as an ideal surface coating for preparing biomimetic NPs.
Different methods for encapsulating nanoparticles in EVs have been reported, such as passive incubation, freeze/thaw cycling, surface conjugation, extrusion, electroporation, and sonication [85,86] Recently, labeling parental cells has appeared as an emerging technique for the fabrication of EV-coated NPs. This method involves direct incubation of parental cells with NPs, cell internalization, incorporation of the NPs into the exosome biogenesis pathway, and exocytosis of NP-loaded EVs to the culture medium [86]. However, the efficacy of this approach is challenging to assess, and there have been few research studies of good quality.
Healthy-Cell-Derived EVs
Macrophages—Macrophage-derived EVs have been examined as potential candidates for producing biomimetic NPs. On this occasion, ultrasmall Ag2S quantum dots (QDs) and doxorubicin hydrochloride (DOX) were simultaneously encapsulated into RAW 264.7-macrophage-secreted vesicles through electroporation to give rise to MVs@QDs&DOX [82]. After 10 min of NIR laser irradiation (808 nm), the shells of macrophage-derived EVs is altered. Then, QDs and DOX can be released for deep tumor penetration of the QDs, producing a synergistic effect of the chemotherapy. One of the advantages of using macrophage-derived EVs is the presence of specific cancer ligands.
Mesenchymal stem cells—mesenchymal-stem-cell-derived EVs have also been explored for encapsulating NIR-responsive nanomaterials. For example, Huang and co-workers synthesized gold nanostars (GNS) with the trans-activating transcriptional (TAT) peptide on the NP surface to increase intracellular uptake in MSCs. TAT-GNS NPs were incubated with MSC for 24 h. Then, released GNS clusters were collected from supernatants of GNS-labeled MSCs that displayed the same protein profile as the unlabeled MSC EVs, confirming encapsulation of TAT-GNS NPs in the EVs.
Cancer-Cell-Derived EVs
As discussed in the previous section, healthy-cell-derived EVs can be decorated by several ligands, such as CD49d protein, that specifically bind to cancer cells and enable cancer homing. However, cancer cells show cell self-recognition; as mentioned above, they possess a natural characteristic, homotypic binding, wherein they tend to adhere to each other, leading to the continuous growth of tumors. Thus, it is reasonable to think that tumor-derived EVs could retain the homotypic targeting capacity, becoming an excellent material for cloaking NPs to increase specific tumor targeting [87]. Although of paramount relevance, this approach is very innovative, and few research efforts have been made. In any case, combinatorial therapies are the current choice for cancer-cell-derived EVs for PTT.
The combination of PTT with immunotherapy is also being investigated. PTT can induce immunogenic cell death, activating dendritic cells (DCs), in which maturation is induced by tumor-associated antigens generated in dying cancer cells after treatment.
CD47 is a transmembrane protein that mainly functions as an anti-phagocytic or “do not eat me” signal. CD47 directly binds with SIRPα, mainly located on macrophages, enabling CD47-expressing cells to evade immune clearance [88]. CT26 cells were genetically engineered to obtain CD47-overexpressed exosomes, fused with thermosensitive liposomes (TRL), and loaded with ICG and R837 (an immune adjuvant) for producing NPs, named I/R@hGLV [84]. The presence of the exosomes covers endowed I/R@hGLV NPs with the ability to target homologous cells, enhancing cellular uptake. At the same time, ICG plus NIR laser irradiation triggered DCs maturation by exposing tumor-associated antigens present in the generated cell debris with the assisted function of R837.
3.2. Nature-Inspired Nanoparticles for Photothermal Therapy
Over the past decades, nanomedicine has been inspired to prepare nanoparticles by natural pathogen infection mechanisms. Viruses, bacteria, and toxins can efficiently evade the immune system while targeting the host cells by specific interactions between the pathogen and cell membrane receptors. Viruses, bacteria, or toxins can also recognize molecules that cancer cells express. Thus, research on pathogen-inspired nanoparticles in the context of cancer has been growing in recent years due to their potential to target specific cancer biomarkers. Besides the considerable potential of this approach, only a few articles have reported the application of PTT using the nature-inspired nanoparticles strategy so far.
3.2.1. Virus-Based Nanoparticles
Virus-like Particles (VLPs)
VLPs are non-replicative and non-infectious protein particles that closely mimic the structure and size of viruses. They comprise self-assembling viral protein subunits or other molecules derived from viruses, which can assemble spontaneously into VLPs without genetic material [89]. VLPs have become an essential tool in vaccine development against viral diseases [90], but are also used in drug delivery, solid tumor targeting, and theranosis [91]. Recently, VLPs have been investigated as potential PTT platforms in cancer treatment. VLPs have been produced using structural proteins derived from various viruses, including human viruses such as immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) [91,92].
Bacteriophages and plant-virus-based nanoparticles (VNP) have also been explored [91,92]. ICG was used for PTT in a bacteriophage-Qβ-based VLP. Bacteriophage Qβ is a small RNA virus that infects bacteria, specifically Escherichia coli. VLPs are expressed in bacteria, specifically bacteriophage Qβ, and incorporate RNA during the assembly process, which affects the adaptive immune response [94].
Virus-Shape-Inspired Nanoparticles
Besides using any of the molecules of the virus for improving nanoparticle cell targeting, researchers have mimicked the morphology of the virus for this purpose. Chen et al. used gold [96], while Wang et al. tuned mesoporous silica nanoparticles to make virus-shaped nanocapsules [97]. Both works demonstrated that the rough surface designed for the virus-like shape nanoparticles enhanced adhesion to promote cellular internalization. Regarding PTT, Tian et al. [98] also showed that raspberry-shaped polypyrrole nanoparticles had superior adsorption at 808 nm and a photothermal conversion effect compared to spherical nanoparticles because of their surface roughness, which enhanced light absorption. Immune responses to the nanocomposites were also dependent on their morphology.
3.2.2. Bacteria-Based Nanoparticles
As previously discussed for eukaryote-derived extracellular vesicles, nanocarriers can also be coated with bacterial membrane vesicles (MVs), such as bacterial outer membrane vesicles (OMVs) released by Gram-negative bacteria [101,102] and Gram-positive-released membrane vesicles referred to as cytoplasmic membrane vesicles (CMVs) [102]. MVs share significant similarities with mammalian EVs in structure and biological activities. Bacterial vesicles have been shown to have a range of biological functions, including cell–cell communication, host–pathogen interactions, and immunomodulation [102]. Thus, biomimetic nanoparticles coated with MVs have shown several potential applications, including drug delivery, immunotherapy, and vaccine development. These nanoparticles are designed to mimic the natural properties of MVs, including their ability to interact with immune cells and trigger an immune response. Additionally, using MVs as a coating material can improve the stability and biocompatibility of the nanoparticles, making them more suitable for in vivo use [101,103].
Besides the promising results as delivery nanocarriers, only a few studies have reported the potential of MV-based NPs for PTT in cancer. Liu and colleagues developed a novel strategy using E. coli bacterial outer membrane vesicles (OMVs) loaded with iron oxide–manganese oxide composite nanoparticles (Fe3O4-MnO2-OMV NPs, FMO) [104]. The FMO particles were designed as a sophisticated multimodal therapy for melanoma treatment, comprising chemotherapy, immunotherapy, and photothermal therapy simultaneously. The iron oxide nanoparticles in FMO generated toxic ROS, while the manganese oxide nanoparticles produced Mn2+ and O2 in the melanoma tumor microenvironment. The Mn2+ acted as an immune adjuvant, while O2 prevented immunosuppression caused by hypoxia and enhanced the immunotherapeutic effect.
3.2.3. Cytotoxic-Protein-Based Nanoparticles
Different natural-derived peptides, venom components, and toxins from various sources have been traditionally used as highly potent cytotoxic drugs for cancer treatment. Nevertheless, over the past few years, an innovative approach has emerged using those proteins for precise therapeutics. Besides their intrinsic cytotoxicity, they can be tuned to recruit various clinically interesting functions, such as specific cell-surface receptor binding. Thus, cytotoxic proteins have started to be applicable in targeted cancer nano therapy as biomimetic systems [106].
On the one hand, cytotoxic-protein-based nanoparticles can be engineered to recognize and bind to receptors overexpressed on the surface of cancer cells [106]. For example, conjugate tumor-targeted cytotoxic nanoparticles have been explored by targeting the chemokine receptor 4 (CXCR4), which is overexpressed in more than 23 human cancers [107]. The cationic peptide T22, an effective CXCR4 ligand, was combined with the active fragments of diphtheria toxin [108,109,110,111], pseudomonas aeruginosa exotoxin [109,111], or the plant toxin ricin [112] to create self-assembling toxin-based nanoparticles. These nanoparticles effectively promoted tumor cell death in vitro and potent CXCR4-dependent anti-tumor effects without systemic toxicity in CXCR4+ tumor mouse models of lymphoma [108,110], colorectal [111], endometrial cancer [109], and acute myeloid leukemia [112]. Despite these promising results, no studies have been reported yet to include photosensitizers in this approach for PTT and combinatorial treatment.
On the other hand, some cytotoxic proteins have intrinsic selectivity for cancer cells, such as Chlorotoxin (CTX), derived from the venom of the scorpion Leiurus quinquestriatus, which has been shown to bind to matrix metalloproteinase-2 (MMP-2) preferentially overexpressed on the surface of neuroectodermal origin [113] and metastatic breast [114] cancer, among others. Different studies reported iron oxide nanoparticles functionalized with CTX to target glioma [115,116,117,118].
4. Conclusions
Cancer is still one of the most significant challenges of our time. Monotherapies are frequently not the best therapeutic option for cancer patients; beyond the three classic pillars of oncology therapeutics (surgery, chemotherapy, radiotherapy) together with modern immuno-therapy, several other exciting options are being developed to bring hope, mainly in the form of combinatorial treatments. One of these options is PTT. Combining NIR-absorbing materials with NIR lasers can locally increase the temperature of specific tissues (tumors). The effect of this temperature increase can be two-fold.
On the one hand, it can directly damage and promote tumoral cell death. At the same time, on the other hand, it can synergize with other treatments by combining therapeutic outcomes and facilitating treatment access to the tumor (for example, by increasing the tumor permeation of a drug or drug delivery system). The specificity of PTT, even in the absence of tumor targeting in the PTT agent provided by the laser, is one of the strong points of PTT as it allows to spare healthy tissues even if the agent accumulates in those. As described in the section above, a few hurdles must be overcome to translate PTT into the clinic.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242015484
This entry is offline, you can click here to edit this entry!
