Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Al–Ni–Er is an essential system in heat-resistant Al alloys. The aluminum-rich corner of this system, which has the most practical application significance. The phase equilibria of the Al–Ni–Er system are investigated via experiments and thermodynamic modeling. The isothermal sections of the Al-rich corner of this ternary system at 600 and 700 °C were determined through equilibrated alloys combined with scanning electron microscopy (SEM), electron probe microanalysis (EPMA) and X-ray diffractometry (XRD).
- Al–Ni–Er system
- equilibrated alloys
- isothermal sections
1. Introduction
Aluminum alloys have a history of nearly a century, but they have developed rapidly. The addition of nickel in aluminum alloys offers higher tensile strength and hardness, greatly improves corrosion resistance [1], and has high specific strength that could effectively reduce the weight of products, lower fuel consumption, and save energy in industrial production [2,3]. It can also promote a reduction in carbon emissions and help in achieving carbon neutrality [4]. The amorphous aluminum alloy has better properties than those of ordinary aluminum alloys. The glass-forming ability (GFA) of Al alloys could be promoted with the addition of rare earth metals (REs) [5,6,7]. Through a comprehensive consideration of cost and performance, we chose the Al–Ni–Er system as our research object.
Phase diagrams and thermodynamics are powerful tools applied to material design [8,9,10,11]. CALPHAD is a more efficient method than experimental research because an experiment cannot cover all points in a 3D phase diagram model, and CALPHAD can obtain comprehensive information that can guide the experiment. Al–Ni–Er is an essential system in heat-resistant Al alloys. Studying its thermodynamics can not only guide the design of Al–Ni–Er alloys, but also supplement the database of Al-based alloys.
2. Binary Al–Ni System
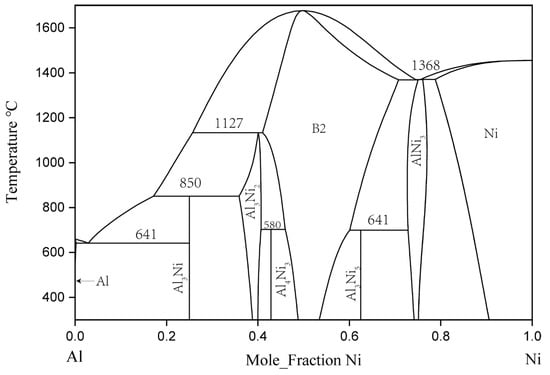
In 1993, Okamoto [12] revised Nash’s [13] evaluation of the binary Al–Ni system, and rereported the experimental phase diagram of the system. The binary Al–Ni phase diagram evaluated by Okamoto is the generally accepted version. In the binary Al–Ni system, there are two terminal solid solutions with an FCC structure, the three intermetallic compounds of NiAl3, Ni2Al3, and Ni5Al3, and an NiAl phase with a B2-ordered structure and an Ni3Al phase with an L12-ordered structure. AlNi, Al3Ni2, and AlNi3 had a certain range of solid solubility at 600 and 700 °C [14].
Since Gwyer [15] initiated his work, many experiments have been carried out to determine the phase equilibrium and thermodynamic quantities of the Al–Ni system. Kaufman, and Nesor and Ansara et al. [16] produced a thermodynamic model of the whole system through phase diagram calculation. Then, Du [17], Ansara [16], Huang [18], Dupin [19], Lu [20], and Chen [21] used different models to optimize this system. The calculated phase diagram and thermodynamic description of Al–Ni were also constantly updated. The final thermodynamic model and parameters of Al–Ni binary system can be found in the thermodynamic simulation of the ternary Al–Cr–Ni system by Dupin et al. [19]. Figure 1 is the Al–Ni binary phase diagram drawn according to the thermodynamic parameters in the literature.
3. Binary Er–Ni System
The phase equilibrium relation of the binary Er–Ni system was first studied by Buschow [23] in 1968. In total, 11 intermetallic compounds were identified: ErNi3, Er2Ni7, ErNi4, Er4Ni17, Er5Ni22, ErNi5, Er2Ni17, Er3Ni, Er5Ni3, ErNi, and ErNi2. In 1974, Moreau et al. [24] studied the Er3Ni2 compound and found that Er5Ni3 should be replaced by Er3Ni2. Subsequently, in 1999, Du et al. [25] used the CALPHAD method to assess thermodynamic parameters and established a comprehensive thermodynamic database of this binary system. The phase diagram of the Er–Ni binary system calculated by Du et al. is shown in Figure 2 [25].
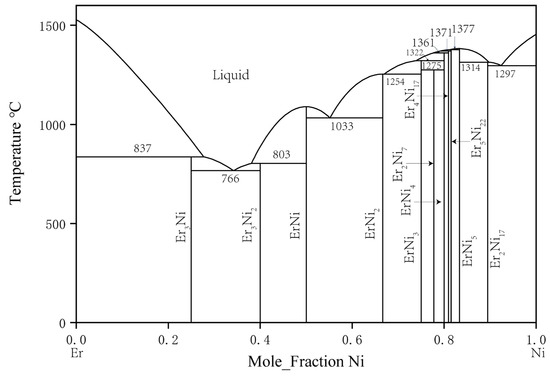
Figure 2. Er–Ni phase diagram assessed by Du et al. [25].
4. Binary Al–Er System
In 1965, The binary Al–Er system was first studied by Buschow and Vucht [26]. They discovered AlEr2, Al2Er3, AlEr, Al2Er, and Al3Er. Their study indicated that the solid solubility of Al in Er was close to 8 at. % at 860 °C, which seemed unusually high. In 1988, Gschneidner and Calderwood [27] restudied this system. Considering the 23% size difference between Er and Al, the actual solid solubility had to be less than 1 at. %. The previous observation might have been due to an impurity effect or nonequilibrium conditions. However, in their results, the curvature change in the liquidus curve did not conform to convention in the 50 to 80 at. % Er composition range. In 2002, Cacciamani et al. [28] conducted thermodynamic modeling and optimization for the Al–Er system, and their Al–Er phase diagram is shown in Figure 3. In 2022, L Xu et al., thermodynamically assessed this system [29].
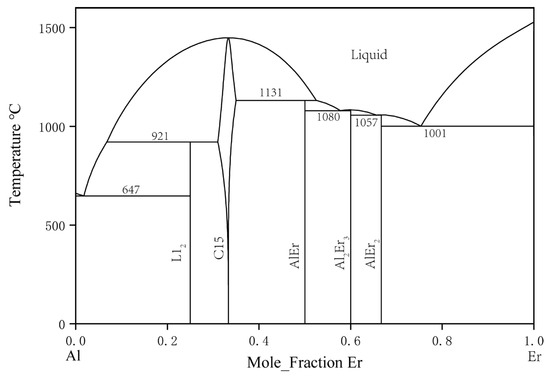
Figure 3. Calculated Al–Er phase diagram by Cacciamani et al. [28].
5. Ternary Al–Ni–Er System
There is relatively little information about the ternary Al–Ni–Er system. In 1982, Zarechnyuk et al. [30] first reported that the Er content in this system was 0–33 at. %. At the isothermal cross section at 800 °C, nine intermediate compounds were determined: τ4–Al4NiEr, τ5–Al3–xNi2+xEr, τ6–Al2NiEr, τ7–AlNi8Er3, τ8–Al2Ni6Er3, τ9–AlNi2Er2, τ10–AlNiEr, Al7Ni3Er2, and Al16Ni3Er. Combined with the later findings regarding the τ2–Al9Ni3Er phase, the Al2Ni3Er phase was reported by Gladyshevskii [31], and Sorgic et al. [32]. Riccardo et al. [33] drew a relatively complete isothermal section phase diagram of the ternary Al–Ni–Er system at 800 °C, as shown in Figure 4.
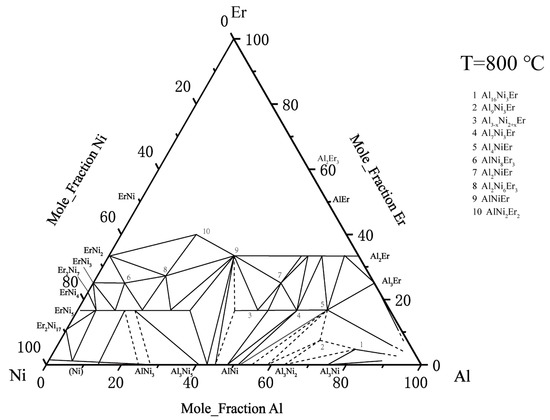
Figure 4. Al–Ni–Er phase diagram assessed by Riccardo et al. [33].
On the basis of the above research, Zhao et al. [34] used the equilibrium alloy method to determine most of the phase diagram of the ternary Al–Ni–Er system at 600 °C with Er content from 0 to 67 at. %. Three new ternary mesophase, namely, AlNiEr4, AlNi6Er13 and AlNi2Er were named τ11, τ12, and τ13. Because the pure phase was not obtained in the experiment, the structures of these ternary compounds were not discussed. The above work also revised ErNi5 τ5. Al2Er, ErNi2, and τ10. The isothermal section of the ternary Al–Ni–Er system at 600 °C was drawn as shown in Figure 5.
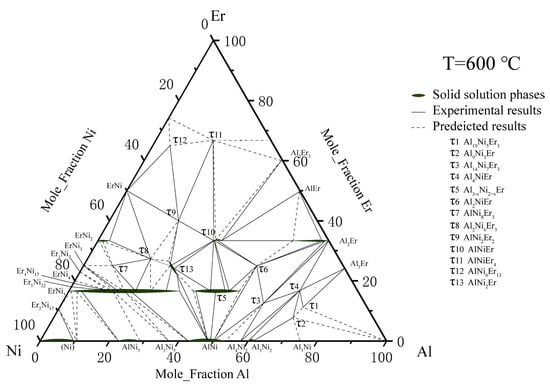
Figure 5. Al–Ni–Er phase diagram assessed by Zhao et al. [34].
Similarly, Zhao et al. measured the isothermal section of the Al–Ni–Er system at 700 °C and 6 phase zones at the Al-rich corner: Al2Er + Al3Er + τ4, Al + τ1 + τ2, Al + τ2 + Al3Ni, Al3Ni2 + τ2 + Al3Ni, Al + τ1 + Al3Er, and τ1 + Al3Er + τ4. At the Al-rich corner (60–100% Al) of the experimental phase diagram of the ternary Al–Ni–Er alloy, at the margin, there were four ternary intermetallic compounds, namely, τ1, τ2, τ3, τ4, and four binary compounds, Al3Ni2, Al3Ni, Al2Er and Al3Er.
According to the literature research, Al2Er should show a certain solid solubility to Ni in a parallel direction to Er. The ideal stoichiometric composition, solid-phase crystal structure, and lattice parameters of the above compounds could be obtained by investigating the existing literature data, as shown in Table 1.
Table 1. Crystallographic data of all phases in the Al–Ni–Er system.
| System | Phase | Prototype | Pearson Symbol | Lattice Parameters (nm) | Refs. | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Al | Fcc_A1, (Al) | Cu | cF4 | 0.40496 | [35] | ||
| Ni | Fcc_A1, (Ni) | Cu | cF4 | 0.3524 | [35] | ||
| Er | Hcp_A3, (Er) | Mg | hP2 | 0.359 | 0.555 | [35] | |
| Al–Ni | Al3Ni | Al3Ni | oP16 | 0.6613 | 0.7367 | 0.4811 | [36] |
| Al3Ni2 | Al3Ni2 | hP5 | 0.4028 | 0.4811 | [37] | ||
| AlNi | CsCl | cP2 | 0.28872 | [35] | |||
| Al3Ni5 | Pt5Ga3 | oC16 | 0.753 | 0.661 | 0.376 | [35] | |
| AlNi3 | Cu3Au | cP4 | 0.35792 | [35] | |||
| Al4Ni3 | Ga4Ni3 | cI112 | 1.144 | [22] | |||
| Ni–Er | Er2Ni17 | Th2Ni17 | hP38 | 0.8287 | 0.8017 | [22] | |
| Er5Ni22 | hP108 | 0.4862 | 7.177 | [22] | |||
| Er4Ni17 | hP126 | 0.4869 | 8.407 | [22] | |||
| ErNi4 | PuNi4 | mS30 | 0.4855 | 0.8444 | 1.0231 | [22] | |
| Er2Ni7 | Gd2Co7 | hR54 | 0.4909 | 3.6067 | [38] | ||
| ErNi3 | PuNi3 | hR36 | 0.4941 | 2.4252 | [30] | ||
| ErNi2 | MgCu2 | cF24 | 0.71175 | [31] | |||
| Er3Ni2 | Er3Ni2 | hR45 | 0.8472 | 1.5680 | [39] | ||
| ErNi | FeB | oP8 | 0.6977 | 0.4110 | 0.5443 | [39] | |
| Er3Ni | Fe3C | oP16 | 0.6804 | 0.9432 | 0.6245 | [40] | |
| Al–Er | Al3Er | AuCu3 | cP4 | 0.4235 | [26] | ||
| Al2Er3 | Al2Zr3 | tP20 | 0.81323 | 0.75039 | [41] | ||
| AlEr2 | Co2Si | oP12 | 0.6516 | 0.5015 | 0.9279 | [42] | |
| AlEr | AlDy | oP16 | 0.55791 | 1.1277 | 0.5574 | [43] | |
| Al–Ni–Er | |||||||
| τ1 | Al19Ni5Er3 | Al119Ni5Gd3 | oS108 | 4.0565 | 15.8906 | 26.9752 | [24] |
| τ2 | Al9Ni3Er | Al9Ni3Er | hR78 | 7.2716 | 27.346 | [44] | |
| τ3 | Al14Ni7Er3 | Al14Ni7Gd3 | hP72 | 17.8776 | 4.03212 | [25] | |
| τ4 | Al4NiEr | YNiAl4 | oS24 | 4.044 | 15.08 | 6.631 | [22] |
| τ5 | Al3–xNi2+xEr | Al3Ni2Y | hP18 | 9.01 | 4.049 | [22] | |
| τ6 | Al2NiEr | MgCuAl2 | oS16 | 4.064 | 10.06 | 6.898 | [22] |
| τ7 | AlNi8Er3 | Ce3Co8Si | hP24 | 5.002 | 15.99 | [22] | |
| τ8 | Al2Ni6Er3 | Ce3Ni6Si2 | cI44 | 8.88 | [22] | ||
| τ9 | AlNi2Er2 | W2CoB2 | oI10 | 5.347 | 8.374 | 4.157 | [37] |
| τ10 | AlNiEr | AlNiZr | hP9 | 6.970 | 3.8003 | [45] | |
| τ11 | AlNiEr4 | [34] | |||||
| τ12 | AlNi6Er13 | [34] | |||||
| τ13 | AlNi2Er | [34] | |||||
| τ14 | Al12Ni2Er3 | This work | |||||
This entry is adapted from the peer-reviewed paper 10.3390/pr11041061
This entry is offline, you can click here to edit this entry!