Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microbial compost plays a crucial role in improving soil health, soil fertility, and plant biomass. These biofertilizers, based on microorganisms, offer numerous benefits such as enhanced nutrient acquisition (N, P, and K), production of hydrogen cyanide (HCN), and control of pathogens through induced systematic resistance. Additionally, they promote the production of phytohormones, siderophore, vitamins, protective enzymes, and antibiotics, further contributing to soil sustainability and optimal agricultural productivity.
- biofertilizer
- plant diseases
- compost
- nutrient transformation
- PGPR
1. Introduction
In recent years, global food security has been increasingly threatened by the combination of population increase and the scarcity of limited arable land worldwide [1][2]. Consequently, the need to boost crop productivity has become a significant challenge in order to meet the demands of a continuously increasing global population [3]. To enhance crop productivity, various chemical fertilizers have been extensively employed worldwide; however, this widespread use has led to the deterioration of both human health and environmental ecology with significant severity [4][5]. Biofertilizers offer a promising solution in order to counteract the harms associated with chemical fertilizers. They are an essential component of integrated nutrient management, contributing significantly to crop output and food security in the agricultural sector [6][7]. Biofertilizers consist of one or more beneficial microbes capable of colonizing the interior or exterior of plants after soil application, as seeds, or as direct exposure on the plants. This colonization facilitates the enhancement of nutrient supply to the host plant, effectively promoting its growth [8][9]. These microbe-containing fertilizers improve the soil fertility through different mechanisms including atmospheric nitrogen fixation, solubilization of unavailable nutrients (such as phosphate, zinc, potassium, and iron), and synthesis of phytohormones [10]. Thus, the biofertilizers improve soil fertility and crop yield by harvesting the natural biological system of nutrients and recycling them [11]. The efficiency and efficacy of biofertilizers has remained controversial in terms of their application in the field and their potential to replace the chemical fertilizers, mainly due to the heterogeneous nature of soil, adaptability to harsh ecological conditions, and unsuitable formulations and carrier materials [12]. Thus, if a nutrient-rich carrier material with potential to facilitate microbial growth is used, outcomes from biofertilizers can be substantially enhanced and the use of chemical fertilizers can be completely or partially cut down. Nowadays, as a result of intensive agricultural activities, a huge amount of organic waste is generated by agricultural fields, and the disposal of this poses difficulties [13].
Production of nutrient-rich compost from agricultural waste has been emerging as an alternative way of disposal [14]. A dark brown or black earthy matter, rich in micro- and macronutrients and produced as a result of aerobic decomposition of biodegradable waste is known as compost [15]. Compost not only enhances the soil fertility in terms of micro- and macronutrients, it also improves the soil architecture by improving water- and air-holding capacity for better root growth. Moreover, compost can play an important role in the bioeconomy because its production does not depend on finite inputs [16]. This technique of waste disposal has proved to be very economic, as it enhances plant nutrient uptake, soil organic matter content, crop yield, and soil biophysical parameters [13][17]. The application of compost has been proven beneficial, as it has good impact on environment and soil quality [18].
Composting is the process in which organic materials like food scraps, leaves, twigs, lawn clippings, wood waste, and other organic matter are decayed by the soil microorganisms under controlled ambiance [15][19]. To carry out the decomposition process, either a pit can be dug in the ground, or the process can be conducted in special vessels called compost bins [20]. Certain factors, including temperature, proper aeration, pH, the quality and quantity of feedstock, etc., must be considered before initializing the process of composting to make it more efficient and reliable [15]. Another key factor to be considered is the ratio between carbon and nitrogen (C/N), which plays an essential function in the composting process. The application of compost enhances the physico-chemical properties of the soil and also exerts a positive impact on the soil’s microbial diversity [21]. The widespread adoption of compost in agriculture faces constraints due to its extended time of action and comparatively reduced nutrient supply to crops when compared with chemical fertilizers [17]. Various reports have been published on the composting of agricultural feedstock and its potential application in improving soil fertility and plant growth [22][23]. Moreover, the potential role of different phytobeneficial microbial communities to the process of composting has also been elucidated by several researchers around the world [24][25].
2. Biofertilizers and Their Advantages
Agricultural productivity is decreasing continuously due to nutrient deficiency in the soils and the growth of obnoxious weeds and pests [26]. Conversely, the production cost has been raised over the last two decades. As a result of these factors, the growth rate in agriculture is falling behind the rapid pace of population growth [27][28]. The population explosion around the globe makes the use of chemicals fertilizers for improving crop production in order to meet food requirements inevitable [29]. Frequent soil tillage, chemical fertilizers, and narrow crop rotations are some of the intensive agricultural techniques that increase production in a short time. However, over time, these techniques have led to a decline in soil organic carbon, soil aggregation strength, and biodiversity, consequently reducing the productivity of field crops. Additionally, they contribute to air and groundwater pollution [30][31]. In this scenario, the biofertilizers have a great potential to improve soil fertility, thus reducing the need for the application of chemical fertilizers. Biofertilizer-mediated agricultural practices also result in crops with improved yield [32][33].
Biofertilizers utilize beneficial microorganisms to optimize plant growth by increasing nutrient supply, effectively enhancing overall nutrient availability, and promoting healthier and more robust plant development [34][35]. Generally, the potential of different beneficial microbes, including nitrogen fixers, phosphorus solubilizers, potassium solubilizers, iron mobilizers, as well as the microbes capable of producing phytohormones, is utilized during biofertilizer synthesis which improves the nutrient profile of the soil [36][37]. Subsequently, the microbe-oriented nutrient recycling through biofertilizers enhances the soil organic matter content and maintains the soil health and sustainability, which is ultimately followed by healthy plant growth. Several bacterial species, known as plant-growth-promoting rhizobacteria (PGPR), such as Azotobacter spp., Escherichia coli, Pseudomonas spp., and Bacillus spp., and arbuscular mycorrhizal fungi (AMF), such as Glomus versiforme, Aspergillus awamori, Glomus macrocarpum, and Sclerocystis coremioides, are often exploited in broad spectrum biofertilizers without causing any negative chemical influence on the soils [38][39][40]. Moreover, co-inoculation of PGPR and AMF also results in enhanced plant growth, nutrient uptake, disease tolerance, and resistance to abiotic stress. For example, co-culture of Rhizobium, Azotobacter, and vesicular arbuscular mycorrhiza (VAM) as biofertilizer enhanced the straw and grain yield when applied to wheat plants along with rock phosphate as a phosphate source [41]. Similarly, a mixed culture comprising Thiobacillus thioxidans, Bacillus subtilis, and Saccharomyces sp. was found capable of converting micronutrients into soluble forms such as Mn, Zn, Fe, etc., and making them available to plants [33]. Trichoderma spp. are also known to improve the tolerance of plants to biotic and abiotic stresses by producing enzymes that can detoxify harmful chemicals and by increasing the production of stress-response proteins in the plant [42]. For example, Trichoderma harzianum inoculation improved tomato plants’ tolerance to chilling stress by enhancing physiological, biochemical, and molecular responses [43]. The biofertilizers that achieve nitrogen fixing, i.e., potassium- and phosphate-solubilizing bacterial strains, significantly improve the growth, production, and qualitative characteristics of food crops [44]. Hence, these microbial-based fertilizers can significantly contribute to the establishment of sustainable agriculture systems, playing a pivotal role in achieving this goal.
Overall, the utilization of biofertilizers has been in practice for a considerable period due to their eco-friendly nature and superior cost-efficiency compared with chemical fertilizers. The biofertilizers can also be used to convert complex organic materials into simple compounds, followed by a change in the color and texture of the soil [45][46]. They have the capability to enhance the crop yield by 25–30% and can help the soil fight against dehydration and other soil-borne illnesses [15]. Therefore, to promote agricultural productivity, it is essential to acknowledge the application of biofertilizers [47][48].
3. Microbial Compost
Agricultural waste is now emerging as a compelling and cost-effective resource that can provide organic matter and essential plant nutrients to the soil, effectively supporting crop production [49]. However, raw organic waste cannot be directly applied because it is unsuitable for land and agricultural crops [50]. Hence, composting stands out as one of the most favorable, straightforward, and cost-effective methods employed to treat this type of wastes [51][52]. Compost can be locally produced on the farm and is an attractive technique of waste disposal. It is can recover valuable plant nutrients and improve the soil’s biophysical characteristics, soil organic matter, and crop yield [53][54]. As the nutrients in compost are slowly released depending upon the microbial biomass, the gradual nutrition availability for the plants is ensured [55]. Thus, the utilization of microbial compost aids in preventing nutrients leaching into groundwater and significantly increases soil productivity in the long term. The soil quality can be enhanced by repeated compost applications, which result in the enriching of microorganisms that are beneficial to the soil, an increased total carbon content, an enhanced cation exchange capacity, and a reduction in the abundance of plant-parasitic nematodes [56]. The use of pesticides and herbicides also decreases due to the improved plant resistance against diseases as a result of compost application [57]. The application of compost, along with certain soil microorganisms such as PGPR and AMF, has been proven to effectively boost soil fertility and health [58].
3.1. The Composting Process
The process of controlled decomposition of organic material like agricultural residues is known as composting [59]. A composting process requires the synergistic action of the natural forces that decompose the organic waste into the organic fertilizer to occur in a safe way [60]. The primary raw materials utilized for composting include agricultural waste such as fruit and vegetable waste, domestic kitchen residues, various crop residues (e.g., stover, cobs, and leaves), and different types of manure, e.g., cattle and poultry [61]. Both aerobic and anaerobic bacteria are involved in the composting process. For better a understanding of the degradation processes that occur in compost, characterization and identification of microorganisms is necessary in the composting process. Common PGPR, like Azotobacter spp., Pseudomonas spp., Escherichia coli, and Bacillus spp., and some AMF, such as Aspergillus awamori, Glomus macrocarpum, and Sclerocystis coremioides, were found to be involved in the decomposition process [62][63]. These microorganisms utilize amino acids, sugars, and lipids present in the feedstock as their energy source [64][65]. Composting offers numerous benefits beyond providing a significant release of various nutrient elements for plants. It contributes to soil conditioning, facilitates efficient manure handling, reduces the risks associated with different pollutants and weed seeds, and promotes pathogen destruction through a high-temperature composting processes [66]. A schematic diagram of the composting process has been presented in Figure 1.
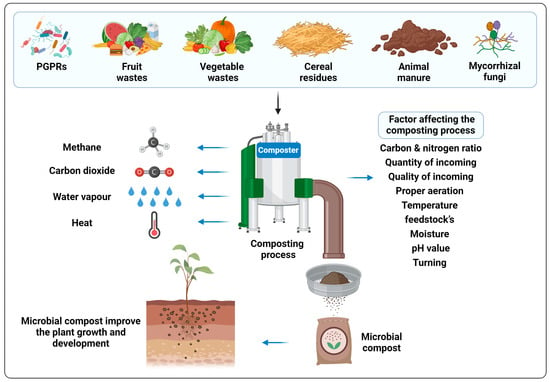
Figure 1. Schematic representation of the composting process.
3.2. The Biochemistry of Composting
The compost feedstock is often rich in organic compounds that are rich in components like carbon (C), hydrogen (H), oxygen (O2), and nitrogen [67]. The lignin, sugars, fats, cellulose, and proteins that are important components of agricultural raw material facilitate the decomposition of organic compounds during the process of composting. These components become progressively more oxidized and form molecules with more functional groups that have, however, a lower molecular weight during the aerobic decomposition [68]. The product obtained after the decomposition of organic matter is known as humus. In addition, the biodegradation occurs under both anaerobic and aerobic circumstances, but the composting process proceeds excellently under aerobic conditions [69]. Many microbes can perform their function in the absence of O2, but the process of anaerobic respiration is less energy-efficient and it utilizes chemical species like sulfate, carbon dioxide, nitrates, sulfur, and oxidized metal ions. Hence, to suppress the development of anaerobic conditions, artificial aerobic conditions should be introduced in the compost pile through pulling or pushing the air [70][71].
3.3. Microorganisms Involved in Plant Growth and the Composting Process
The composting process can be characterized as the biodegradation of organic waste into useful products under controlled ambiance. These products are applied to the soil, and they effectively enhance the physico-chemical properties of the soil [72][73]. The biodegradation of organic waste is accelerated by diverse microbial species belonging to different microbial groups, namely, bacteria, actinomycetes, and filamentous fungi. The bacterial species belonging to the genera Pseudomonas, Bacillus, Paenibacilus, and Enterobacter were found to be the most abundant microorganisms, having a very high population density of 3.0 × 108 CFU/g throughout the composting process, followed by actinomycetes (mainly the species of genus Streptomyces, Nocardia, Micromonospora, Thermomonospora Dactylosporangium, and Kibdelosporangium). The members of filamentous fungi that drive the composting process mainly belong to the genus Aspergillus and have a low population density (i.e., 1.2–1.6 × 108 CFU/g) [72].
A group of specific microorganisms which positively influences plant growth are PGPR and AMF [48][74]. A number of PGPR and AMF, along with their potential roles in plant improvement, are presented in Table 1. These microbes belong to many genera, including Xanthomonas, Agrobacterium, Streptomyces, Alcaligenes, Cellulomonas, Arthrobacter, Amorpho sporangium, Bacillus, Pseudomonas sp., Azotobacter, Actinoplanes, Rhizobium, Erwinia, Bradyrhizobium, Enterobacter, Rhizophagus irregularis, and Glomus intraradices [6]. Generally, the microorganisms that are used as BF are potassium solubilizers, phosphorus solubilizers, nitrogen fixers, and phytohormone producers [75]. Nitrogen-fixing microbes convert atmospheric N2 to ammonia [76]. Some microorganisms belonging to the Ectorhizospheric strains and Endosymbiotic rhizobia have been defined as efficient phosphate solubilizers [7][77]. The most potent strains from bacterial genera that solubilize phosphorus are Pseudomonas, Bacillus, Enterobacter, and Rhizobium genera [9][78]. Similarly, numerous PGPR species belonging to different genera, namely, Bradyrhizobium, Pseudomonas, Rhizobium, Agrobacterium, Klebsiella, Enterobacter, Azotobacter, and Bacillus, are best known for their phytohormones production potential. These microbe-oriented phytohormones have an effective role in plant growth stimulation.
The ability of PGPR to solubilize potassium rock by secreting organic acids has also been investigated. A number of bacterial strains, including Burkholderia sp., Bacillus mucilaginosus, Ferrooxidans sp., Paenibacillus sp., Pseudomonas sp., Bacillus edaphicus, and Acidothiobacillus sp., are PGPR that solubilize potassium-bearing minerals, thereby releasing the potassium in the form that is available for plants [79]. Therefore, utilizing PGPR as biofertilizers to enhance agriculture can reduce the dependence on agrochemicals and promote sustainable crop production (Figure 2).
Table 1. Some PGPR and AMF strains involved in plant growth promotion.
| Chemicals | Microorganisms | Beneficial Effects | References |
|---|---|---|---|
| Direct Mechanism | |||
| Nitrogen fixation | Bradyrhizobium japonicum, Glomus macrocarpum, Azotobacter vinelandii, Bacillus, Rhizobium, Beijerinckiaderxii, Klebsiella pneumoniae, Enterobacter cloacae, Citrobacterfreundii, and Pseudomonas putida | The conversion of atmospheric N2 into plant-utilizable forms triggers improvement in plant development and yield | [80][81][82][83][84][85] |
| Phosphate solubilization | Penicillium brevicompactum, Aspergillus niger, Pseudomonas striata, Enterobacter, Erwinia, Bacillusmegaterium, Ochrobactrumanthropi, Bacilus, Beijerinckia, Burkholderia, Rhizobium, and Serratia | Solubilizing the inorganic phosphorus from insoluble compounds and making them available to the plants | [86][87][88][89][90] |
| Potassium solubilization | Aspergillus niger, Aspergillus terreus, Acidothiobacillus sp., Bacillus edaphicus, Ferrooxidans sp., Bacillus mucilaginosus, Pseudomonas sp., Burkholderia sp., and Paenibacillus sp. | Solubilizing potassium rock by producing and secreting organic acids and making them available to the plants for growth and development | [79][91][92][93] |
| Zinc mobilization | Beauveria caledonica, Hymenoscyphus ericae, Oidiodendron maius Pennisetum glaucum, Gluconacetobacter diazotrophicus, fluorescent pseudomonads, and Bacillus sp. | Solubilizing the insoluble Zn into soluble form and hence having efficient role in plant growth and development | [94][95][96][97][98][99] |
| Production of phytohormones | Paecilomyces formosus, Asprgillus fumigatus, Fusarium proliferatum Azotobacter, Arthrobacter, Azospirillum, Pseudomonas, Bacillus, Acinetobacter, Flavobacterium, Enterobacter, Micrococcus, Agrobacterium, Clostridium, Rhizobium, and Xanthomonas | Play an important role as regulators of growth and development of plants | [100][101][102][103][104][105] |
| Siderophore production | Aspergillus fumigatus, Glomus etunicatum, Glomus mossae, Trichoderma spp., Pseudomonas fluorescens, Rhodococcus, Acinetobacte, and Pseudomonas putida |
Solubilize and sequester iron from the soil and then provide it to the plant cells | [86][106][107][108][109] |
| Exopolysaccharides production | Azotobacter vinelandii, Bacillus drentensis, Enterobacter cloacae, Rhizobium sp., Agrobacterium sp., and Xanthomonas sp. | Plays a pivotal role in increasing the number of soil macropores, aggregating rhizospheric soil particles, and maintaining water potential | [110][111] |
| Indirect Mechanisms | |||
| Hydrogen cyanide | Alcaligenes, Aeromonas, Rhizobium, Pseudomonas, and Bacillus sp. | Powerful inhibitor of many metal enzymes, especially copper-containing cytochrome Coxidases | [112][113][114] |
| ACC deaminase activity | Gigaspora rosea, Achromobacter, Azospirillum, Pseudomonas, Enterobacter, Bacillus, and Rhizobium | Plants were able to tolerate environmental stresses by keeping a normal amount of ethylene in their root zone | [115][116][117] |
| Induced systemic resistance | Trichoderma virens, Pseudomonas, and Bacillus spp. | Induced resistance is the state of an enhanced defensive ability developed by plants when appropriately stimulated | [118][119][120][121] |
| Production of vitamins | Glomus aggregatum, Glomus viscosum, Azotobacter vinelandii, Azospirillum brasilense, Azospirillum spp., Pseudomonas fluorescens, Rhizobium leguminosarum, Rhizobium etli, Sinorhizobium meliloti, Mesorhizobium loti, and Bacillus subtilis | Facilitate the production of essential compounds for plants and bacteria, induce resistance against pathogens, and directly promote plant growth | [122][123][124] |
| Production of protective enzymes | Aspergillus niger, Glomus spp., Bacillus, Burkholderia, Enterobacter, Pseudomonas, Serratia, and Staphylococcus | May have a dramatic effect on the cycling of nutrients such as phosphorus, nitrogen, and sulfur | [125][126][127] |
| Production of antibiotics | Pseudomonas, Bacillus, and Azotobacter | Prevent the detrimental effects of pathogens on plants through production of inhibitory substances | [128][129] |
| Volatile organic compound (VOCs) | Bacillus amyloliquefaciens, Bacillus subtilis, Pseudomonas fluorescens, Bacillus mojavensis, Trichoderma spp., Trametes gibbosa, and Trametes versicolor | VOCs have a significant role in the plant growth promotion and suppression of plant diseases | [130][131][132][133] |
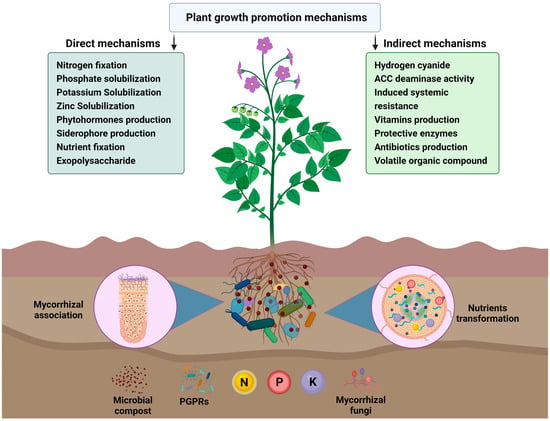
Figure 2. Schematic representation of the compost-based biofertilizers. Mycorrhizal fungal filaments and plant-growth-promoting rhizobacteria in the soil act as to support the development of the plant root system and are more effective at water and nutrient absorption than the roots themselves. PGPR and AMF also explore the soil and reach places unattainable to roots and increase nutrient uptake by plants from the soil. Their effects on plant growth through two different mechanisms, such as direct mechanism and indirect mechanism, are illustrated.
4. The Formulation of the Microbial Compost-Based Biofertilizers
Enriching compost with nutrients, beneficial bacteria, and AMF is a key strategy for improving its nutritional value and enhancing its positive effects on plant growth [134]. By establishing a mutualistic relationship, soil microorganisms and plants work together to facilitate nutrient uptake without disrupting the overall physico-chemical or biological equilibrium of the system. Endophytic PGPR, for example, resides within the plant roots, positively impacting the plants through the secretion of phytohormones, nitrogen fixation, enhanced phosphorus uptake, and the solubilization of inorganic phosphates. This cooperative partnership promotes healthier plant growth and fosters a sustainable ecosystem [135][136]. In general, supplementation of compost with different types of nutrients as well as its bioaugmentation with potential PGPR and AMF strains can lead to value addition in BF technology. Thus, such compost-based biofertilizers need to be added in future long-term farming strategies in order to make the farm yield more sustainable and cost-effective. For this purpose, the compost can be developed through natural processes followed by adding the inoculum of known PGPR or AMF strain(s) at an optimal population density. However, the survival of PGPR or AMF strain(s) in the compost and their synergistic effect, along with natural microflora of compost, are vital factors, which need to be assessed before developing compost-based biofertilizers.
This entry is adapted from the peer-reviewed paper 10.3390/plants12203550
References
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335.
- Suryanto, S.; Trinugroho, I.; Susilowati, F.; Aboyitungiye, J.B.; Hapsari, Y. The Impact of Climate Change, Economic Growth, and Population Growth on Food Security in Central Java Indonesia. Nat. Environ. Pollut. Technol. 2023, 22, 1017–1022.
- Ahmed, T.; Noman, M.; Gardea-Torresdey, J.L.; White, J.C.; Li, B. Dynamic interplay between nano-enabled agrochemicals and the plant-associated microbiome. Trends Plant Sci. 2023.
- Soleimani, H.; Mansouri, B.; Kiani, A.; Omer, A.K.; Tazik, M.; Ebrahimzadeh, G.; Sharafi, K. Ecological risk assessment and heavy metals accumulation in agriculture soils irrigated with treated wastewater effluent, river water, and well water combined with chemical fertilizers. Heliyon 2023, 9, e14580.
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of uncontrolled fertilization and heavy metal toxicity associated with arsenic (As), lead (Pb) and cadmium (Cd), and possible remediation. Toxicology 2022, 477, 153274.
- Mohammadi, K.; Sohrabi, Y. Bacterial biofertilizers for sustainable crop production: A review. ARPN J. Agric. Biol. Sci. 2012, 7, 307–316.
- Shahid, M.; Hameed, S.; Imran, A.; Ali, S.; van Elsas, J.D. Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J. Microbiol. Biotechnol. 2012, 28, 2749–2758.
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206.
- Shahid, M.; Hameed, S.; Tariq, M.; Zafar, M.; Ali, A.; Ahmad, N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann. Microbiol. 2015, 65, 1525–1536.
- Mazid, M.; Khan, T.A. Future of bio-fertilizers in Indian agriculture: An overview. Int. J. Agric. Food Res. 2015, 3, 10–23.
- Pandey, J.; Singh, A. Opportunities and constraints in organic farming: An Indian perspective. J. Sci. Res. 2012, 56, 47–72.
- Wang, Y.; Liu, Z.; Hao, X.; Wang, Z.; Wang, Z.; Liu, S.; Tao, C.; Wang, D.; Wang, B.; Shen, Z. Biodiversity of the beneficial soil-borne fungi steered by Trichoderma-amended biofertilizers stimulates plant production. NPJ Biofilms Microbiomes. 2023, 9, 46.
- Ahmad, R.; Jilani, G.; Arshad, M.; Zahir, Z.A.; Khalid, A. Bio-conversion of organic wastes for their recycling in agriculture: An overview of perspectives and prospects. Ann. Microbiol. 2007, 57, 471–479.
- Moretti, S.M.L.; Bertoncini, E.I.; Abreu-Junior, C.H. Composting sewage sludge with green waste from tree pruning. Sci. Agric. 2015, 72, 432–439.
- Baruah, S.; Srivastava, S. Production and Assesment of Compost as a Bio-Fertilizer Using Kitchen Waste from NIT Rourkela Hostels and Study of Comparative Plant Growth. Ph.D. Thesis, National Institute of Technology, Rourkela, India, 2015.
- Ingrao, C.; Bacenetti, J.; Bezama, A.; Blok, V.; Geldermann, J.; Goglio, P.; Koukios, E.G.; Lindner, M.; Nemecek, T.; Siracusa, V. Agricultural and forest biomass for food, materials and energy: Bio-economy as the cornerstone to cleaner production and more sustainable consumption patterns for accelerating the transition towards equitable, sustainable, post fossil-carbon societies. J. Clean. Prod. 2016, 117, 4–6.
- Singh, A.; Manna, M. Solid Waste Management Through Vermi-and Phosphate-Enriched Composting Techniques for Sustainable Agriculture. In Water Quality Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 399–406.
- Oyetunji, O.; Bolan, N.; Hancock, G. A comprehensive review on enhancing nutrient use efficiency and productivity of broadacre (arable) crops with the combined utilization of compost and fertilizers. J. Environ. Manag. 2022, 317, 115395.
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 162024.
- El-Desuki, M.; Hafez, M.M.; Mahmoud, A.R.; El-Al, A.; Faten, S. Effect of organic and bio fertilizers on the plant growth, green pod yield, quality of pea. Int. J. Acad. Res. 2010, 2, 87–92.
- Tumuhairwe, J.B.; Tenywa, J.S. Bacterial community changes during composting of municipal crop waste using low technology methods as revealed by 16S rRNA. Afr. J. Environ. Sci. Technol. 2018, 12, 209–221.
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153.
- Li, Z.; Lu, H.; Ren, L.; He, L. Experimental and modeling approaches for food waste composting: A review. Chemosphere 2013, 93, 1247–1257.
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485.
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562.
- Rehman, A.D.; Ayyub, M.; Zahir, Z.A. The trend towards suppressing most troublesome weeds for sustainable production of wheat. In Food and Agriculture on Social, Economic and Environmental Linkages; Iksad Publications: Ankara, Türkiye, 2023; p. 74.
- Jat, S.L.; Suby, S.; Parihar, C.M.; Gambhir, G.; Kumar, N.; Rakshit, S. Microbiome for sustainable agriculture: A review with special reference to the corn production system. Arch. Microbiol. 2021, 203, 2771–2793.
- Sambo, U.; Sule, B. Impact of Climate Change on Food Security in Northern Nigeria. Green Low-Carbon Econ. 2023.
- Babu, S.; Rana, D.; Yadav, G.; Singh, R.; Yadav, S. A review on recycling of sunflower residue for sustaining soil health. Int. J. Agron. 2014, 2014, 601049.
- Chen, J.-H. The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. In Proceedings of the International Workshop on Sustained Management of the Soil-Rhizosphere System for Efficient Crop Production and Fertilizer Use, Bangkok, Thailand, 16–20 October 2006; p. 20.
- Zhang, J.; Hamza, A.; Xie, Z.; Hussain, S.; Brestic, M.; Tahir, M.A.; Ulhassan, Z.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Arsenic transport and interaction with plant metabolism: Clues for improving agricultural productivity and food safety. Environ. Pollut. 2021, 290, 117987.
- Son, T.; Thu, V.; Duong, V.; Hiraoka, H. Effect of Organic and Bio-Fertilizers on Soybean and Rice Cropping System; Japan International Research Center for Agricultural Sciences: Tsukuba, Ibaraki, Japan, 2007.
- Bhattacharjee, R.; Dey, U. Biofertilizer, a way towards organic agriculture: A review. Afr. J. Microbiol. Res. 2014, 8, 2332–2343.
- de Moraes, A.C.P.; Ribeiro, L.d.S.; de Camargo, E.R.; Lacava, P.T. The potential of nanomaterials associated with plant growth-promoting bacteria in agriculture. 3 Biotech 2021, 11, 318.
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 606815.
- Bhattacharyya, P.; Jha, D. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350.
- Mourouzidou, S.; Ntinas, G.K.; Tsaballa, A.; Monokrousos, N. Introducing the Power of Plant Growth Promoting Microorganisms in Soilless Systems: A Promising Alternative for Sustainable Agriculture. Sustainability 2023, 15, 5959.
- Chunhaleuchanon, S. Screening of rhizobacteria for their plant growth promoting activities. KMITL Sci. Technol. J. 2008, 8, 18–23.
- Jelin, J.; Dhanarajan, M.; Mariappan, V. Assessment of compost as a bio-fertilizer for the growth of paddy. J. Environ. Biol. 2013, 34, 975.
- Sadhana, B. Arbuscular Mycorrhizal Fungi (AMF) as a Biofertilizer-a Review. Int. J. Curr. Microbiol. App. Sci 2014, 3, 384–400.
- Kachroo, D.; Razdan, R. Growth, nutrient uptake and yield of wheat (Triticum aestivum) as influenced by biofertilizers and nitrogen. Indian J. Agron. 2006, 51, 37–39.
- Fazeli-Nasab, B.; Shahraki-Mojahed, L.; Piri, R.; Sobhanizadeh, A. Trichoderma: Improving growth and tolerance to biotic and abiotic stresses in plants. In Trends of Applied Microbiology for Sustainable Economy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 525–564.
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141.
- Youssef, M.; Eissa, M. Biofertilizers and their role in management of plant parasitic nematodes. A review. E3 J. Biotechnol. Pharm. Res 2014, 5, 1–6.
- Adesemoye, A.O.; Kloepper, J.W. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12.
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Abid, M.; Ullah, S. Effects of organic and inorganic manures on maize and their residual impact on soil physico-chemical properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32.
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Salamatinia, B.; Gholami, Z.; Zwain, H.M. A review on composting of oil palm biomass. Environ. Dev. Sustain. 2015, 17, 691–709.
- Tariq, M.; Noman, M.; Ahmed, T.; Hameed, A.; Manzoor, N.; Zafar, M. Antagonistic features displayed by Plant Growth Promoting Rhizobacteria (PGPR): A Review. J. Plant Sci. Phytopathol. 2017, 1, 38–43.
- Tariq, M.; Jameel, F.; Ijaz, U.; Abdullah, M.; Rashid, K. Biofertilizer microorganisms accompanying pathogenic attributes: A potential threat. Physiol. Mol. Biol. Plants 2022, 28, 77–90.
- Wang, Y.; Liu, Y.; Feng, W.; Zeng, S. Waste Haven Transfer and Poverty-Environment Trap: Evidence from EU. Green Low-Carbon Econ. 2023, 1, 41–49.
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87.
- Lima, J.Z.; da Silva, E.F.; Patinha, C.; Rodrigues, V.G.S. Sorption and post-sorption performances of Cd, Pb and Zn onto peat, compost and biochar. J. Environ. Manag. 2022, 321, 115968.
- Viaene, J.; Van Lancker, J.; Vandecasteele, B.; Willekens, K.; Bijttebier, J.; Ruysschaert, G.; De Neve, S.; Reubens, B. Opportunities and barriers to on-farm composting and compost application: A case study from northwestern Europe. Waste Manag. 2016, 48, 181–192.
- Nkonya, E.; Kato, E.; Kabore, C. Impact of Farmer-Managed Natural Regeneration on Resilience and Welfare in Mali. Green Low-Carbon Econ. 2023.
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. Biochar Environ. Manag. Sci. Technol. Implement. 2015, 2, 421–454.
- Stirling, G.R. Biological control of plant-parasitic nematodes. In Diseases of Nematodes; CRC Press: Boca Raton, FL, USA, 2017; pp. 103–150.
- Yadav, S.; Babu, S.; Yadav, M.; Singh, K.; Yadav, G.; Pal, S. A review of organic farming for sustainable agriculture in Northern India. Int. J. Agron. 2013, 2013, 718145.
- Rengalakshmi, R.; Manjula, M.; Prabavathy, V.; Jegan, S.; Selvamukilan, B. Building Bioeconomy in Agriculture: Harnessing Soil Microbes for Sustaining Ecosystem Services. In Towards a Sustainable Bioeconomy: Principles, Challenges and Perspectives; Springer: Berlin/Heidelberg, Germany, 2018; pp. 261–277.
- Qu, F.; Wu, D.; Li, D.; Zhao, Y.; Zhang, R.; Qi, H.; Chen, X. Effect of Fenton pretreatment combined with bacterial inoculation on humification characteristics of dissolved organic matter during rice straw composting. Bioresour. Technol. 2022, 344, 126198.
- Harindintwali, J.D.; Zhou, J.; Yu, X. Lignocellulosic crop residue composting by cellulolytic nitrogen-fixing bacteria: A novel tool for environmental sustainability. Sci. Total Environ. 2020, 715, 136912.
- Meyer, A.; Ehimen, E.; Holm-Nielsen, J. Future European biogas: Animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenergy 2017, 111, 154–164.
- Avery, L.M.; Booth, P.; Campbell, C.; Tompkins, D.; Hough, R.L. Prevalence and survival of potential pathogens in source-segregated green waste compost. Sci. Total Environ. 2012, 431, 128–138.
- Akhtar, M.J.; Asghar, H.N.; Shahzad, K.; Arshad, M. Role of plant growth promoting rhizobacteria applied in combination with compost and mineral fertilizers to improve growth and yield of wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 381–390.
- Gil, M.; Carballo, M.; Calvo, L. Fertilization of maize with compost from cattle manure supplemented with additional mineral nutrients. Waste Manag. 2008, 28, 1432–1440.
- Shilev, S.; Naydenov, M.; Vancheva, V.; Aladjadjiyan, A. Composting of food and agricultural wastes. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: Boston, MA, USA, 2007; pp. 283–301.
- Chatterjee, N.; Flury, M.; Hinman, C.; Cogger, C.G. Chemical and Physical Characteristics of Compost Leachates. A Review; Washington State University: Pullman, WA, USA, 2013.
- Guo, X.-x.; Liu, H.-t.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899.
- Meunchang, S.; Panichsakpatana, S.; Weaver, R.W. Inoculation of sugar mill by-products compost with N 2-fixing bacteria. Plant Soil 2005, 271, 219–225.
- Zanella, A.; Ponge, J.-F.; Guercini, S.; Rumor, C.; Nold, F.; Sambo, P.; Gobbi, V.; Schimmer, C.; Chaabane, C.; Mouchard, M.-L. Humusica 2, article 16: Techno humus systems and recycling of waste. Appl. Soil Ecol. 2018, 122, 220–236.
- Mata-Alvarez, J.; Mace, S.; Llabres, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16.
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583.
- Chen, C.-Y.; Mei, H.-C.; Cheng, C.-Y.; Lin, J.-H.; Chung, Y.-C. Enhancing the conversion of organic waste into biofertilizer with thermophilic bacteria. Environ. Eng. Sci. 2012, 29, 726–730.
- Lin, C.; Cheruiyot, N.K.; Hoang, H.-G.; Le, T.-H.; Tran, H.-T.; Bui, X.-T. Benzophenone biodegradation and characterization of malodorous gas emissions during co-composting of food waste with sawdust and mature compost. Environ. Technol. Innov. 2021, 21, 101351.
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881.
- Gothwal, R.; Nigam, V.; Mohan, M.; Sasmal, D.; Ghosh, P. Screening of nitrogen fixers from rhizospheric bacterial isolates associated with important desert plants. Appl. Ecol. Eviron. Res. 2008, 6, 101–109.
- Bakulin, M.; Grudtsyna, A.; Pletneva, A.Y. Biological fixation of nitrogen and growth of bacteria of the genus Azotobacter in liquid media in the presence of perfluorocarbons. Appl. Biochem. Microbiol. 2007, 43, 399.
- Igual, J.; Valverde, A.; Cervantes, E.; Velázquez, E. Phosphate-solubilizing bacteria as inoculants for agriculture: Use of updated molecular techniques in their study. Agronomie 2001, 21, 561–568.
- Satyaprakash, M.; Nikitha, T.; Reddi, E.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. App. Sci 2017, 6, 2133–2144.
- Liu, D.; Lian, B.; Dong, H. Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol. J. 2012, 29, 413–421.
- James, E.K.; Gyaneshwar, P.; Barraquio, W.L.; Mathan, N.; Ladha, J.K. Endophytic diazotrophs associated with rice. In The Quest for Nitrogen Fixation in Rice; International Rice Research Institute: Makati City, Philippines, 2000; pp. 119–140.
- Meunchang, S.; Panichsakpatana, S.; Weaver, R. Tomato growth in soil amended with sugar mill by-products compost. Plant Soil 2006, 280, 171–176.
- Meng, L.; Zhang, A.; Wang, F.; Han, X.; Wang, D.; Li, S. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 2015, 6, 339.
- Bulgarelli, R.G.; Marcos, F.C.C.; Ribeiro, R.V.; de Andrade, S.A.L. Mycorrhizae enhance nitrogen fixation and photosynthesis in phosphorus-starved soybean (Glycine max L. Merrill). Environ. Exp. Bot. 2017, 140, 26–33.
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. 2010, 107, 13754–13759.
- Mehmood, S.; Khatoon, Z.; Amna; Ahmad, I.; Muneer, M.A.; Kamran, M.A.; Ali, J.; Ali, B.; Chaudhary, H.J.; Munis, M.F.H. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2023, 69, 197–211.
- Chakraborty, U.; Chakraborty, B.; Basnet, M.; Chakraborty, A. Evaluation of Ochrobactrum anthropi TRS-2 and its talc based formulation for enhancement of growth of tea plants and management of brown root rot disease. J. Appl. Microbiol. 2009, 107, 625–634.
- Rodriguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555.
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. A proposal for isolating and testing phosphate-solubilizing bacteria that enhance plant growth. Biol. Fertil. Soils 2013, 49, 1–2.
- Altaf, M.M.; Imran, M.; Abulreesh, H.H.; Khan, M.S.; Ahmad, I. Diversity and Applications of Penicillium spp. in Plant-Growth Promotion. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–276.
- Rossati, K.F.; Figueiredo, C.C.d.; Mendes, G.d.O. Aspergillus niger Enhances the Efficiency of Sewage Sludge Biochar as a Sustainable Phosphorus Source. Sustainability 2023, 15, 6940.
- Teotia, P.; Kumar, V.; Kumar, M.; Shrivastava, N.; Varma, A. Rhizosphere Microbes: Potassium Solubilization and Crop Productivity–Present and Future Aspects. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 315–325.
- Ahmad, M.; Nadeem, S.M.; Naveed, M.; Zahir, Z.A. Potassium-solubilizing bacteria and their application in agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 293–313.
- Prajapati, K.; Sharma, M.; Modi, H. Isolation of two potassium solubilizing fungi from ceramic industry soils. Life Sci. Leafl. 2012, 5, 71–75.
- Fomina, M.; Alexander, I.J.; Hillier, S.; Gadd, G. Zinc phosphate and pyromorphite solubilization by soil plant-symbiotic fungi. Geomicrobiol. J. 2004, 21, 351–366.
- Martino, E.; Perotto, S.; Parsons, R.; Gadd, G.M. Solubilization of insoluble inorganic zinc compounds by ericoid mycorrhizal fungi derived from heavy metal polluted sites. Soil Biol. Biochem. 2003, 35, 133–141.
- Kumar, V.; Sarma, M.; Saharan, K.; Srivastava, R.; Kumar, L.; Sahai, V.; Bisaria, V.; Sharma, A. Effect of formulated root endophytic fungus Piriformospora indica and plant growth promoting rhizobacteria fluorescent pseudomonads R62 and R81 on Vigna mungo. World J. Microbiol. Biotechnol. 2012, 28, 595–603.
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 2016, 7, 615.
- Rokhbakhsh-Zamin, F.; Sachdev, D.; Kazemi-Pour, N.; Engineer, A.; Pardesi, K.R.; Zinjarde, S.; Dhakephalkar, P.K.; Chopade, B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011, 21, 556–566.
- Sharma, S.K.; Sharma, M.P.; Ramesh, A.; Joshi, O.P. Characterization of zinc-solubilizing Bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol. 2012, 22, 352–359.
- Tsavkelova, E.; Klimova, S.Y.; Cherdyntseva, T.; Netrusov, A. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126.
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503.
- Akhtar, M.; Siddiqui, Z. Effects of phosphate solubilizing microorganisms and Rhizobium sp. on the growth, nodulation, yield and root-rot disease complex of chickpea under field condition. Afr. J. Biotechnol. 2009, 8, 3489–3496.
- Waqas, M.; Khan, A.L.; Shahzad, R.; Ullah, I.; Khan, A.R.; Lee, I.-J. Mutualistic fungal endophytes produce phytohormones and organic acids that promote japonica rice plant growth under prolonged heat stress. J. Zhejiang Univ.-Sci. B 2015, 16, 1011–1018.
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.-J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127.
- Kumar, V.; Prasher, I. Phosphate solubilization and indole-3-acetic acid (IAA) produced by Colletotrichum gloeosporioides and Aspergillus fumigatus strains isolated from the rhizosphere of Dillenia indica L. Folia Microbiol. 2023, 68, 219–229.
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276.
- Patel, D.; Patel, S.; Thakar, P.; Saraf, M. Siderophore Producing Aspergillus spp as Bioinoculant for Enhanced Growth of Mung Bean. Int. J. Adv. Agric. Sci. Technol. 2017, 6, 111–120.
- Li, Y.; Wang, Z.; Liu, X.; Song, Z.; Li, R.; Shao, C.; Yin, Y. Siderophore biosynthesis but not reductive iron assimilation is essential for the dimorphic fungus Nomuraea rileyi conidiation, dimorphism transition, resistance to oxidative stress, pigmented microsclerotium formation, and virulence. Front. Microbiol. 2016, 7, 931.
- Happacher, I.; Aguiar, M.; Yap, A.; Decristoforo, C.; Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus: Impact on biotic interactions and potential translational applications. Essays Biochem. 2023, 67, 829–842.
- Hindersah, R. Exopolysaccharide-Producing Azotobacter for Bioremediation of Heavy Metal-Contaminated Soil. In Advances in Agricultural and Industrial Microbiology; Volume-2: Applications of Microbes for Sustainable Agriculture and In-Silico Strategies; Springer: Singapore, 2022; pp. 103–117.
- Çam, S.; Bicek, S. The effects of temperature, salt, and phosphate on biofilm and exopolysaccharide production by Azotobacter spp. Arch. Microbiol. 2023, 205, 87.
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38.
- El-Rahman, A.; Shaheen, H.A.; El-Aziz, A.; Rabab, M.; Ibrahim, D.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control 2019, 29, 41.
- Sneka, M.; Shanmitha, A.; Priyadharshini, A.; Thilakavathi, G.; Joselin, J.; Sarenya, R.; Nachiar, T.; Kaleeswari, G.; Pushpakanth, P.; Tamilselvi, S. Isolation and characterization of Rhizobium from green gram (Vigna radiata). Curr. Agric. Res. J. 2022, 10, 277–289.
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth. FEMS Microbiol. Ecol. 2008, 64, 459–467.
- Duan, J.; Müller, K.M.; Charles, T.C.; Vesely, S.; Glick, B.R. 1-aminocyclopropane-1-carboxylate (ACC) deaminase genes in rhizobia from southern Saskatchewan. Microb. Ecol. 2009, 57, 423–436.
- Ghosh, S.; Penterman, J.N.; Little, R.D.; Chavez, R.; Glick, B.R. Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of canola, Brassica campestris. Plant Physiol. Biochem. 2003, 41, 277–281.
- Pastori, G.M.; Kiddle, G.; Antoniw, J.; Bernard, S.; Veljovic-Jovanovic, S.; Verrier, P.J.; Noctor, G.; Foyer, C.H. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 2003, 15, 939–951.
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448.
- Djonović, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889.
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882.
- Karunakaran, R.; Ebert, K.; Harvey, S.; Leonard, M.; Ramachandran, V.; Poole, P. Thiamine is synthesized by a salvage pathway in Rhizobium leguminosarum bv. viciae strain 3841. J. Bacteriol. 2006, 188, 6661–6668.
- Palacios, O.A.; Bashan, Y.; de-Bashan, L.E. Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—An overview. Biol. Fertil. Soils 2014, 50, 415–432.
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L. AM fungi and PGP Pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193.
- Pawar, P.M.A.; Derba-Maceluch, M.; Chong, S.L.; Gómez, L.D.; Miedes, E.; Banasiak, A.; Ratke, C.; Gaertner, C.; Mouille, G.; McQueen-Mason, S.J. Expression of fungal acetyl xylan esterase in Arabidopsis thaliana improves saccharification of stem lignocellulose. Plant Biotechnol. J. 2016, 14, 387–397.
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31.
- Albalawi, M.A.; Abdelaziz, A.M.; Attia, M.S.; Saied, E.; Elganzory, H.H.; Hashem, A.H. Mycosynthesis of silica nanoparticles using Aspergillus niger: Control of Alternaria solani causing early blight disease, induction of innate immunity and reducing of oxidative stress in eggplant. Antioxidants 2022, 11, 2323.
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Kumar, A. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 2013, 51, 282–286.
- Chaiharn, M.; Chunhaleuchanon, S.; Lumyong, S. Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J. Microbiol. Biotechnol. 2009, 25, 1919–1928.
- Rath, M.; Mitchell, T.; Gold, S. Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent. Microbiol. Res. 2018, 208, 76–84.
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83.
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017, 29, 67–75.
- Raza, W.; Ling, N.; Liu, D.; Wei, Z.; Huang, Q.; Shen, Q. Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol. Res. 2016, 192, 103–113.
- Bhantana, P.; Rana, M.S.; Sun, X.-c.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37.
- Paul, E. 1-Soil Microbiology, Ecology, and Biochemistry in Perspective; Elsevier: Amsterdam, The Netherlands, 2007.
- Iqbal, S.; Hameed, S.; Shahid, M.; Hussain, K.; Ahmad, N.; Niaz, M. In vitro characterization of bacterial endophytes from tomato (Solanum lycopersicum L.) for phytobeneficial traits. Appl. Ecol. Environ. Res. 2018, 16, 1037–1051.
This entry is offline, you can click here to edit this entry!
