Ocular inflammatory diseases, including uveitis, involve inflammation within the eye and can affect various parts of the eye, leading to discomfort, vision impairment, and potentially serious complications if left untreated. The causes of uveitis and ocular inflammatory diseases can vary, including autoimmune disorders, infections (such as viral, bacterial, or fungal), systemic diseases (such as rheumatoid arthritis or inflammatory bowel disease), or sometimes the cause may remain unknown (idiopathic). Symptoms of uveitis and ocular inflammatory diseases may include eye redness, pain, light sensitivity, blurred vision, floaters, and in severe cases, vision loss. Another pathology affecting the middle layer of the eye is uveal melanoma, which is the most common primary intraocular malignancy in adults that develops in the uveal melanocytes. Since these are serious and potentially life-altering diseases, proper diagnosis, treatment, and ongoing monitoring are crucial to effectively manage these conditions and maintain ocular health. In this regard, suprachoroidal injections is being investigated as a new potential treatment options for these conditions.
- SC injection
- Vision preservation
- Inflammatory disease
- Uveal melanoma
- Anticancer activity
- Immune-mediated response
- Ophthalmology
1. Uveitis
Suprachoroidal triamcinolone acetonide (SCTA) has shown positive effects on visual and anatomical outcomes with minimal adverse events (AEs) in treating ME secondary to non-infectious uveitis (NIU) [1]. Goldstein et al. (2016) investigated the efficacy and safety of 4.0 mg SCTA in eight eyes with NIU, observing improvements in BCVA in all treated eyes by 26 weeks. Among the reported AEs (n=38), 89% were mild or moderate, primarily affecting the ocular domain. Notably, uveitis progression, cataract progression requiring extraction, and ocular pain were reported at rates of 18%, 3%, and 16% respectively, with no cases of increased IOP [1].
In a porcine model of acute uveitis induced by lipopolysaccharide injection, Noronha et al. (2015) compared the anti-inflammatory effects of SCTA to oral prednisone. SCTA (2.0 mg) exhibited faster anti-inflammatory effects than oral prednisone (3.0 mg/kg/d) by day 1. SCTA was as effective as high-dose prednisone and superior to low-dose prednisone. On days 1 and 2 of treatment, SCTA showed lower inflammation scores compared to controls, while on day 3, both high-dose prednisone and SCTA had lower inflammation scores than controls. These findings highlight the advantages of local SCTA administration over systemic prednisone, which can lead to long-term side effects [2].
Gilger et al. (2013) compared the efficacy and safety of different doses and administration routes of triamcinolone acetonide (TA) in a porcine model of acute posterior uveitis. They found that 0.2 mg and 2.0 mg subconjunctival TA (SCTA) were equally effective as 2.0 mg intravitreal TA (IVTA) in reducing inflammation, with similar intraocular pressure (IOP) and optical coherence tomography (OCT) measurements. Histologic scores for ocular posterior segment inflammation were significantly lower in the high-dose SCTA group compared to IVTA, as shown in Figure 1. Additionally, the high-dose SCTA group had lower vitreous humor cell count and protein concentration compared to the low-dose SCTA and IVTA groups. No significant differences were observed in aqueous humor protein concentration, and adverse events were reported within 3 days of treatment [3].
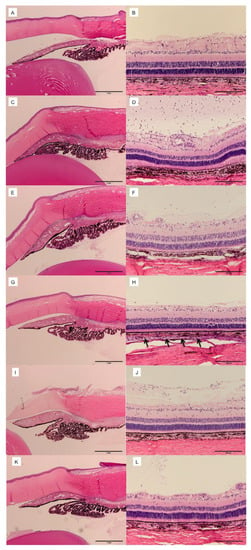
Figure 1. Ocular histopathology of eyes 3 days after IVT injection of balanced salt solution (BSS) or 100 ng of lipopolysaccharide (LPS) and 72 h after SCS or IVT injection of vehicle, 0.2 mg TA (low-dose TA), or 2.0 mg of TA (high-dose TA). Hematoxylin and eosin stain. (A) Anterior segment of eyes injected with BSS IVT and vehicle in SCS (group 1). (B) Posterior segment of eyes injected with BSS IVT and vehicle in SCS (group 1). (C) Anterior segment of eyes injected with LPS IVT and vehicle in SCS (group 2). (D) Posterior segment of eyes injected with LPS IVT and vehicle in SCS (group 2). (E) Anterior segment of eyes injected with LPS IVT and low-dose TA in SCS (group 3). (F) Posterior segment of eyes injected with LPS IVT and low-dose TA in SCS (group 3). (G) Anterior segment of eyes injected with LPS IVT and high-dose TA in SCS (group 4). (H) Posterior segment of eyes injected with LPS IVT and high-dose TA in SCS (group 4). Arrows indicate presence of TA in SCS. (I) Anterior segment of eyes injected with LPS IVT and low-dose TA IVT (group 5). (J) Posterior segment of eyes injected with LPS IVT and low-dose TA IVT (group 5). (K) Anterior segment of eyes injected with LPS IVT and high-dose TA IVT (group 6). (L) Posterior segment of eyes injected with LPS IVT and high-dose TA IVT (group 6). Reproduced with permission [3].
Patel et al. (2013) found that 4.0 mg subconjunctival triamcinolone acetonide (SCTA) was equally effective as intravitreal TA (IVTA) in reducing ocular inflammation in a rabbit model of panuveitis induced by subretinal endotoxin. The study lasted 22 days, and no adverse events, including intraocular pressure (IOP) changes, were reported. SCTA-treated eyes exhibited less panuveitis than IVTA and control eyes after 24 hours. Both SCTA and IVTA significantly reduced vitritis, aqueous flare, cellularity, and histopathological inflammation compared to controls [4].
In a rabbit model of uveitis induced by lipopolysaccharide, Chen et al. (2015) compared the effects of 50 µL (2.0 mg) SCTA to subtenon injection of 20 mg TA. SCTA demonstrated better therapeutic efficacy than subtenon TA, with significantly lower aqueous humor cells, lower vitreous opacity scores, and reduced vitreous inflammation on histology. Following SCTA, there was an acute elevation in IOP, with higher volumes of SCTA leading to higher IOP. The peak concentration of TA (<1.0 ng/mL) was detected in the retina and posterior vitreous, with non-detectable levels in the aqueous and 11.6 ng/mL in the plasma [5].
Porcine models, which share anatomical similarities and retinal vascular patterns with the human eye, have provided valuable insights into subconjunctival injection studies. However, it is important to note that these models represent acute disease and do not fully capture the chronic nature of uveitis, emphasizing the need for human clinical trials. The improvement in BCVA observed in the study by Goldstein et al. led to further exploration of SCTA for managing uveitic macular edema (ME) in the PEACHTREE trial, as discussed earlier. Nonetheless, large-scale, long-term, and masked controlled studies are still needed to evaluate the efficacy and safety of SCTA in different types of uveitis without ME, addressing various aspects of ocular involvement [4].
2. Ocular Inflammatory Diseases
Ketorolac, a short-acting nonsteroidal anti-inflammatory drug (NSAID), is commonly used topically for ocular inflammation and pain relief. However, topical administration may result in suboptimal drug levels or adverse effects (AEs) with increasing dosages, including burning, stinging, delayed corneal healing, and conjunctival hyperemia [192]. Intravenous (IV) injection carries the risk of vitreous opacity and retinal pathologies [6].
In a study with 54 rabbits, Wang et al. (2012) found that IV injection of 250 μg/0.05 mL Ketorolac Tromethamine resulted in higher and longer-lasting intraocular concentrations compared to subconjunctival (SC) and intracameral (IC) injections. Maximum concentrations of Ketorolac in the vitreous and retina-choroid were highest with IV injection, followed by SC and IC injections. IV injection showed a significantly larger amount of Ketorolac in the retina-choroid compared to SC injection. The half-life of Ketorolac was also longer with IV injection, with plasma concentrations below 0.4 μg/mL in all three groups. Ketorolac remained detectable in the retina-choroid for 24 and 8 hours after IV and SC injections, respectively [7].
In another study by Liu et al. (2012), unilateral SC injections of 3.0 mg and 6.0 mg Ketorolac Tromethamine in rabbits were found to be safe compared to controls. Electroretinography showed no abnormal changes at 1 to 4 weeks post-injection, and the histomorphology of retinal cells remained preserved at 4 weeks compared to the control group [8].
Currently, there is limited research on the use of SC Ketorolac for ocular inflammatory diseases. Existing evidence suggests that up to 6.0 mg of SC Ketorolac is safe from functional and anatomical perspectives on the rabbit retina, but larger animal studies are needed to demonstrate efficacy and further safety. Optimization of Ketorolac drug formulations to prolong the duration of action in the subconjunctival space (SCS) requires further investigation. Comparative studies should also assess the efficacy and safety of SC Ketorolac against other ocular delivery methods, including IV injection, retrobulbar injection, and topical administration, for various inflammatory diseases in larger, multi-center human trials. Interestingly, studies have explored IV injection of Ketorolac, among other NSAIDs, in the treatment of conditions such as cystoid macular edema (CME), diabetic macular edema (DME), choroidal neovascularization (CNV), uveitis, and age-related macular degeneration (AMD) [9][10][11][12]. Given the favorable AE profile of NSAIDs compared to corticosteroids, particularly regarding cataract progression and intraocular pressure (IOP) elevation, further research on SC injection is warranted.
3. Uveal melanoma
AU-011, also known as Belzupacap Sarotalocan, shows promise as a treatment for ocular melanoma. When activated by light, this compound induces cellular necrosis through an immune-mediated response. In rabbit models of choroidal melanoma, AU-011 demonstrated effective anticancer activity by distributing well in the choroid and remaining active for several days. Tumor regression and cancer cell necrosis were observed histologically [13].
In the same rabbit model, SC injection of AU-011 outperformed IV injection in terms of tumor distribution and bioavailability, reducing unintended exposure. SC injection resulted in higher tumor penetration levels compared to IV injection, with sustained presence up to 48 hours. IV injection primarily kept AU-011 on the tumor surface, while SC administration led to negligible levels in the vitreous and high exposure in the tumor and choroid-retina [14]. In a study focused on intraocular melanoma, suprachoroidal injection of resin beads and fluorescent microspheres using a microcatheter successfully delivered the agents without causing an inflammatory reaction [15].
AU-011 shows promise as an alternative to radiotherapy in preventing vision loss in choroidal melanoma. IV AU-011 injection in a multi-center trial demonstrated good tolerability, adequate tumor control, and vision preservation [16]. In the same trial, SC AU-011 injection was also found to be safe in the dose escalation phase, with manageable adverse events (AEs) such as anterior chamber inflammation, conjunctival hyperemia, eye pain, and punctate keratitis [17].
Brachytherapy, the current standard of treatment for uveal melanoma, is associated with various ocular complications and radiation toxicity [18]. Animal studies have shown that SC injection allows for targeted drug delivery, quick onset of action, and reduced risk of AEs, potentially expanding the range of treatable tumor sizes. The upcoming efficacy results of the clinical trial by Demirci and colleagues hold the potential to revolutionize choroidal melanoma treatment [19]. However, ocular AEs can still occur with SC injection, and larger multicenter studies with longer follow-ups are necessary to assess safety and long-term remission rates. Future randomized controlled trials comparing radiotherapy, IV AU-011, and SC AU-011 could determine the most effective and safe treatment for uveal melanoma patients.
References
- Goldstein, D.A.; Do, D.; Noronha, G.; Kissner, J.M.; Srivastava, S.K.; Nguyen, Q.D. Suprachoroidal Corticosteroid Administration: A Novel Route for Local Treatment of Noninfectious Uveitis. Transl. Vis. Sci. Technol. 2016, 5, 14.
- Noronha, G.; Blackwell, K.; Gilger, B.C.; Kissner, J.; Patel, S.R.; Walsh, K.T. Evaluation of Suprachoroidal CLS-TA and Oral Prednisone in a Porcine Model of Uveitis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3110.
- Gilger, B.C.; Abarca, E.M.; Salmon, J.H.; Patel, S. Treatment of Acute Posterior Uveitis in a Porcine Model by Injection of Triamcinolone Acetonide into the Suprachoroidal Space Using Microneedles. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2483–2492.
- Patel, S.; Carvalho, R.; Mundwiler, K.; Meschter, C.; Verhoeven, R. Evaluation of Suprachoroidal Microinjection of Triamcinolone Acetonide in a Model of Panuveitis in Albino Rabbits. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2927.
- Chen, M.; Li, X.; Liu, J.; Han, Y.; Cheng, L. Safety and Pharmacodynamics of Suprachoroidal Injection of Triamcinolone Acetonide as a Controlled Ocular Drug Release Model. J. Control. Release 2015, 203, 109–117.
- Kompella, U.B.; Kadam, R.S.; Lee, V.H.L. Recent Advances in Ophthalmic Drug Delivery. Ther. Deliv. 2010, 1, 435–456.
- Wang, M.; Liu, W.; Lu, Q.; Zeng, H.; Liu, S.; Yue, Y.; Cheng, H.; Liu, Y.; Xue, M. Pharmacokinetic Comparison of Ketorolac after Intracameral, Intravitreal, and Suprachoroidal Administration in Rabbits. Retina 2012, 32, 2158–2164.
- Liu, S.; Liu, W.; Ma, Y.; Liu, K.; Wang, M. Suprachoroidal Injection of Ketorolac Tromethamine Does Not Cause Retinal Damage. Neural Regen. Res. 2012, 7, 2770–2777.
- Maldonado, R.M.; Vianna, R.N.G.; Cardoso, G.P.; de Magalhães, A.V.; Burnier, M.N. Intravitreal Injection of Commercially Available Ketorolac Tromethamine in Eyes with Diabetic Macular Edema Refractory to Laser Photocoagulation. Curr. Eye Res. 2011, 36, 768–773.
- Giannantonio, C.; Papacci, P.; Purcaro, V.; Cota, F.; Tesfagabir, M.G.; Molle, F.; Lepore, D.; Baldascino, A.; Romagnoli, C. Effectiveness of Ketorolac Tromethamine in Prevention of Severe Retinopathy of Prematurity. J. Pediatr. Ophthalmol. Strabismus 2011, 48, 247–251.
- Margalit, E.; Boysen, J.L.; Zastrocky, J.P.; Katz, A. Use of Intraocular Ketorolac Tromethamine for the Treatment of Chronic Cystoid Macular Edema. Can. J. Ophthalmol. J. Can. Ophtalmol. 2010, 45, 409–410.
- Kim, S.J.; Toma, H.S. Inhibition of Choroidal Neovascularization by Intravitreal Ketorolac. Arch. Ophthalmol. 2010, 128, 596–600.
- Savinainen, A.; Grossniklaus, H.; Kang, S.; Rasmussen, C.; Bentley, E.; Krakova, Y.; Struble, C.B.; Rich, C. Ocular Distribution and Efficacy after Suprachoroidal Injection of AU-011 for Treatment of Ocular Melanoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3615.
- Savinainen, A.; Grossniklaus, H.E.; King, S.; Wicks, J.; Rich, C.C. Ocular Distribution and Exposure of AU-011 after Suprachoroidal or Intravitreal Administration in an Orthotopic Rabbit Model of Human Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2861.
- Kang, S.J.; Patel, S.R.; Berezovsky, D.E.; Zhang, Q.; Yang, H.; Grossniklaus, H.E. Suprachoroidal Injection of Microspheres with Microcatheter in a Rabbit Model of Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1459.
- Mruthyunjaya, P.; Schefler, A.C.; Kim, I.K.; Bergstrom, C.; Demirci, H.; Tsai, T.; Bhavsar, A.R.; Capone, A.; Marr, B.; McCannel, T.A.; et al. A Phase 1b/2 Open-Label Clinical Trial to Evaluate the Safety and Efficacy of AU-011 for the Treatment of Choroidal Melanoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4025.
- Demirci, H.; Narvekar, A.; Murray, C.; Rich, C. 842P A Phase II Trial of AU-011, an Investigational, Virus-like Drug Conjugate (VDC) for the Treatment of Primary Indeterminate Lesions and Small Choroidal Melanoma (IL/CM) Using Suprachoroidal Administration. Ann. Oncol. 2022, 33, S934.
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and Adverse Events of Plaque Brachytherapy for Ocular Melanoma. J. Contemp. Brachyther. 2019, 11, 392–397.
- Aura Biosciences. A Phase 2 Open-Label, Ascending Single and Repeat Dose Escalation Trial of Belzupacap Sarotalocan (AU-011) via Suprachoroidal Administration in Subjects with Primary Indeterminate Lesions and Small Choroidal Melanoma; Aura Biosciences: Boston, MA, USA, 2023.
