1. Introduction
The level of difficulty for plastic biodegradation depends on the chemical properties of the target plastics and the availability of plastic-degrading microorganisms and enzymes. Thus, the development of biodegradation technologies for different types of plastics has progressed at varying rates. Numerous microorganisms exhibiting plastic degradation capabilities have been identified in diverse environments [
8]. Moreover, the degradation ability of these microorganisms/enzymes is evaluated using various methods such as the following: (1) using scanning electron microscopy (SEM), atomic force microscopy (AFM), and water contact angle (WCA) analysis to monitor the change in surface structure and hydrophobicity [
9,
10]; (2) using a universal mechanical testing system to assess changes in mechanical properties [
11]; (3) simple weighing to investigate weight loss [
12]; (4) Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and nuclear magnetic resonance (NMR) to identify the formation of new functional groups [
9,
12]; (5) cell growth tests using plastic as a sole carbon source [
10]; and (6) using high-performance liquid chromatography and gas chromatography–mass spectrometry (GC-MS) to monitor chemicals generated from plastic degradation [
13]. This section summarizes the highest biodegradation performances achieved by enzymes (
Table 1) and microbial strains (
Table 2) for fossil-based plastics (polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polyurethane (PU), polyethylene terephthalate (PET), polystyrene (PS), polylactic acid (PLA), and polybutylene succinate (PBS)).
Table 1. Plastic-degrading enzymes with the highest degradation performance.
Table 2. Plastic-degrading microorganisms with the highest degradation performance.
2. Fossil-Based Plastics
2.1. Polyethylene and Polypropylene
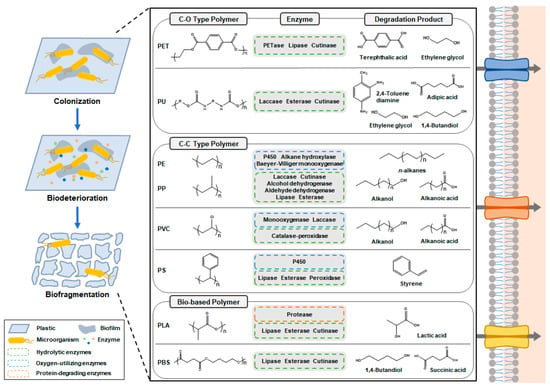
The degradation of PE and PP proceeds at an exceptionally slow rate in the natural environment. This can be attributed primarily to the robust structural stability and hydrophobic characteristics of these polymers, owing to their backbone chains consisting exclusively of C-C and C-H bonds. Due to the challenging nature of their degradation, our understanding of the routes, mechanisms, and necessary enzymes involved in PE and PP degradation remains at an early stage. The degradation of PE and PP is envisioned to occur in three stages: (1) The first stage is the formation of hydrolyzable functional groups in the C-C backbones of PE and PP. Alkane hydroxylases [
17] and cytochrome P450 [
10] are potential enzymes that can hydroxylate PE and PP to initiate biodegradation (
Figure 2 and
Table 1) [
51]. In addition, enzymes including Baeyer–Villiger monooxygenases, alcohol dehydrogenases, and aldehyde dehydrogenases can contribute to increasing the proportion of oxygen in the backbone of PE and PP [
8]. (2) The second stage is the hydrolysis and fragmentation of the carbon chain. Classes of enzymes such as cutinases [
14], lipases [
15], and esterases [
16] are potential candidates for hydrolyzing hydroxylated PE and PP into shorter-chain alkanoic acids and alkanols (
Figure 2 and
Table 1) [
8]. These processes in steps (1) and (2) are iterated until the chain length of the degradation products of PE and PP becomes sufficiently short for microbial utilization. (3) The third stage is the assimilation of degradation products for microbial biomass formation and other metabolic pathways. While most microorganisms prefer carbon chains shorter than C10, some microorganisms, such as the
Acinetobacter sp. M-1 strain, have been reported to utilize C13-C44 alkanes as a sole carbon source [
52].
Figure 2. Enzymatic degradation of various types of plastics. Plastic-degrading microorganisms colonize the plastic surface and release enzymes for biodeterioration and biofragmentation.
Despite the highly recalcitrant nature of PE and PP, there have been numerous reports of various bacterial, fungal, and insect species exhibiting PE degradation capabilities [
8]. However, very few reports exist on successful plastic degradation to a degree where these materials can serve as a sole carbon source in metabolic pathways. In a recent study, the
Bacillus thuringiensis JNU01 strain, isolated from a landfill site, was identified to grow from an OD600 (optical density at 600 nm) of 0.2 to 1 after 30 days in M9 minimal medium containing PE powder as a sole carbon source (
Table 1) [
10]. GC-MS analysis of the culture medium identified the production of PE degradation products, including C7-C29 alkanes, C9 alkene, C11 acid, C6 alcohol, and C11-C15 ethers. Moreover, FTIR, H-NMR, and XPS analyses confirmed the formation of hydroxyl, carboxyl, and amide groups within the degraded PE sample. Notably, sequence analysis and transcriptional expression profiling led to the discovery of a cytochrome P450 catalyzing the hydroxylation of PE (
Table 1). In another study, the
Pseudomonas aeruginosa WGH-6 strain, isolated from landfill soil, exhibited growth, increasing from an OD600 of 0.2 to 0.35 after 10 days when cultivated in a minimal salt medium containing PP powder as a sole carbon source (
Table 2) [
35]. Extended cultivation for 40 days resulted in 17.2% weight loss in the PP particles, and SEM images revealed the formation of cracks and pits on the surface of the PP particles. WCA analysis indicated a reduced contact angle (56.88°) compared to untreated PP (108.22°), confirming the PP degradation activity. Furthermore, GC-MS analysis of the culture medium identified the predominant production of C29-C35 alkanes as PP degradation products.
2.2. Polyvinylchloride
The degradation of PVC poses significant challenges due to PVC’s hydrophobic nature and the absence of readily hydrolyzable ester bonds. As a result, the present state of research into PVC biodegradation remains in its early stages, and thus far, no specific enzymes have been identified for degrading PVC. PVC degradation is proposed to occur in four stages: (1) The first stage is the oxidative dechlorination of PVC. Enzymes such as non-heme chloroperoxidases, perhydrolases, and haloacid dehalogenase-like hydrolases have been identified as potential catalysts capable of facilitating the oxidative dechlorination of halogenated compounds [
53]. (2) The second stage is the increased formation of hydrolyzable functional groups in dechlorinated PVC. Enzymes like catalase-peroxidases, monooxygenases, dioxygenases, laccases, alcohol dehydrogenases, and aldehyde dehydrogenases may play a role in enhancing the oxygen content in the PVC backbone [
53]. (3) The third stage is the hydrolysis and fragmentation of oxygenated PVC. Enzymes such as esterases, dihydroxy acid dehydratases, and lipases have the potential to depolymerize oxygenated PVC into shorter compounds, including alcohols, mono-acids, and di-acids (
Figure 2) [
40]. (4) The fourth stage is the assimilation of degradation products into microbial biomass formation and other metabolic pathways.
Numerous studies have reported on the possibility of PVC biodegradation by various fungal and bacterial species [
54,
55,
56]. However, there is a scarcity of reports detailing the effective degradation of PVC to a level where it can serve as a sole carbon source in metabolic pathways. In a recent study, the
Klebsiella sp. EMBL-1 strain isolated from a larval intestine was reported to form a biofilm on the surface of a PVC film and grow from an OD600 of 0.2 to 0.6 after 10 days in a mineral salt medium (
Table 2) [
40]. Over the course of 90 days of cultivation, SEM images revealed the formation of cracks and pits on the PVC film surface, and 19.57% weight loss was observed in the PVC film.
GC-MS analysis identified the production of PVC degradation products, including C5-C24 alkanols, alkanoic acids, and ethers. Furthermore, FTIR and H-NMR analyses confirmed the formation of hydroxyl and ethane groups within the degraded PVC film. These findings were corroborated by WCA analysis, which indicated a reduced contact angle (76.1°) compared to untreated PVC film (86.3°). Finally, multi-omics studies and enzymatic assays identified the involvement of a catalase-peroxidase enzyme in PVC depolymerization. These findings furnish a pivotal cornerstone, offering valuable insights into the potential structure of the PVC degradation pathway.
2.3. Polyurethane
The level of difficulty in microbial degradation of PES-PU and PE-PU varies depending on the chemical structures of these plastics. In contrast to PES-PU, the ether groups in the carbon chain of PE-PU make degradation much more difficult due to strong hydrolysis resistance [
57]. Hence, most PU degradation studies have focused on PES-PU. The ester groups in the carbon chain of PES-PU make it susceptible to hydrolytic degradation [
58]. PES-PU degradation using enzyme classes such as lipases, esterases, cutinases, proteases, and laccases has been reported in numerous studies (
Table 1) [
59]. Among those enzymes, esterases from
P. chlororaphis [
60] and
Comamonas acidovorans TB-35 [
61] which preferably hydrolyze the ester bonds in aliphatic and aromatic polyesters, releasing carboxylic acids and alcohols, have been well studied for their protein structures and molecular dynamics [
62]. Few reports exist on PES-PU degradation sufficient for use in metabolic pathways as a sole carbon source. In a study,
C. acidovorans TB-35 isolated from soil was reported to grow up to an OD580 of 2.48 after 7 days in a mineral salt medium while consuming 50 mg of PES-PU as a sole carbon source (
Table 2) [
41]. The main PES-PU degradation products are composed of adipic acid, 1,4-butanediol (1,4-BDO), 2,4-toluenediamine (2,4-TDA), and ethylene glycol (EG) (
Figure 2) [
63].
The soft segments of PES-PU, corresponding to the polyol part, are predicted to biodegrade after the oxidation of the α-methylene hydrogen atom in polyether. However, no enzymes that degrade the soft segment of PES-PU have been reported up to now. The hard segments of PES-PU, corresponding to the isocyanate part, are reported to be degraded by
Staphylococcus epidermis urease via urea bond hydrolysis [
64]. In another study, the
Rhodococcus equi TB-60 strain isolated from soil was reported to grow in a minimal salt medium containing toluene-2,4-dicarbamic acid dibutyl ester (TDCB), a compound with a chemical structure similar to PES-PU [
42]. This bacterium degraded 2.1 mM of TDCB in 10 days and was identified to produce 2,4-TDA and carbamic acid butyl ester as degradation products using GC-MS. Furthermore, urethane hydrolase from the
R. equi TB-60 strain was determined to hydrolyze aliphatic and aromatic urethane compounds (
Table 2).
2.4. Polyethylene Terephthalate
The identification of PET-degrading microorganisms, including
Thermobifida fusca,
Fusarium solani pisi, and
Ideonella sakaiensis, has played a substantial role in advancing the field of PET biodegradation and has paved the way for PET recycling [
65]. Since the initial discovery of these microorganisms, numerous native enzymes have been identified and further engineered to enhance the efficiency of PET degradation. PET is known to degrade through the following pathway: (1) PET hydrolyzes to bis(2-hydroxyethyl) terephthalate (BHET), mono(2-hydroxyethyl) terephthalate (MHET), TPA, and EG. Enzymes classes such as PETases, cutinases, leaf-branch compost cutinase (LCC), lipases, hydrolases, and esterases are known to hydrolyze PET (
Table 1) [
22,
23,
66]. Among these enzymes, a recent study applied rational engineering techniques, such as molecular docking and the analysis of enzyme contact surfaces, to enhance the catalytic activity of LCC [
23]. The engineered LCC, with residue changes at F243W/D238C/S283C/Y127G, exhibited exceptional PET degradation capability, producing TPA at a rate of 16.7 g/L/h using 3 mg of enzymes per g of PET, representing the highest reported performance in PET degradation to date. (2) BHETase hydrolyzes BHET to MHET, TPA, and EG. Until recently, heterogeneous degradation products (MHET, BHET, TPA, EG) were obtained from PET degradation due to the absence of BHETase. This resulted in reduced product yield and compromised physical properties in recycled PET synthesis [
67]. However, a recent study identified two BHETases from the
B. subtilis PET-86 and
Chryseobacterium sp. PET-29 strains capable of hydrolyzing BHET (
Table 1) [
26]. This discovery of BHETase completes the PET biodegradation pathway and opens the possibility for fully bio-based PET recycling. (3) MHET is hydrolyzed to TPA and EG. While the enzymes described in step (1) have little effect on MHET hydrolysis, MHETase derived from
I. sakaiensis has been identified to hydrolyze MHET with high substrate specificity (
Table 1) [
43]. Given its crucial role in complete PET degradation, extensive studies have been conducted on the crystal structure and catalytic mechanism of MHETase [
68]. These findings have paved the way for substantial efforts in protein engineering of MHETase to enhance its catalytic performance and enable the co-utilization of MHET and BHET as substrates [
27]. (4) TPA and EG are assimilated into microbial biomass formation and other metabolic pathways. A study reported that both the
P. putida KT2440 and JM37 strains can grow using EG as a sole carbon source [
69]. These strains of
P. putida have the ability to convert EG into glyoxylate, which is then incorporated into the central carbon network to support biomass formation. Furthermore, another study unveiled the discovery of the novel
Comamonas sp. E6 strain, which possesses the capability to grow using TPA as the sole carbon source [
70]. This finding marks the first instance of a strain with such a unique ability. The
Comamonas sp. E6 strain possesses the ability to convert TPA into protocatechuic acid (PCA), which subsequently enters the PCA 4,5-cleavage pathway, contributing to biomass synthesis.
2.5. Polystyrene
While the complete degradation mechanisms and pathways of PS remain a subject of ongoing research, degradation pathways have been proposed based on studies involving PS-degrading microorganisms (
Table 2) [
44,
45]: (1) The first is the cleavage of the main chain of PS into aromatic monomers, such as styrene. Enzymes such as lipases, esterases, P450, and peroxidases are hypothesized to cleave the main chain of PS (
Table 1) [
71]. (2) The second is the conversion of styrene into acetyl-CoA. (3) The third is the utilization of acetyl-CoA in the tricarboxylic acid (TCA) cycle for microbial biomass formation and other metabolic pathways.
Until recently, there have been no reports on the degradation of PS mediated by microorganisms. In a study, the
P. aeruginosa DSM 50071 strain isolated from the gut of
Zophobas atratus, a Styrofoam-eating larva, was identified for the first time to grow in a carbon-free basal solid medium using PS as a sole carbon source (
Table 2) [
44]. After 60 days of cultivation, the SEM image of the PS particle surface showed edge smoothing and the formation of holes. These findings were consistent with the results of WCA analysis, indicating a reduced contact angle of 79.8° compared to untreated PS (91.56°). Moreover, XPS and FTIR analyses revealed the formation of hydroxyl and carbonyl groups, as well as a reduction in C-C bonds, providing evidence for PS biodegradation. In another study, the
A. johnsoniii JNU01 strain, isolated from soil, exhibited PS-concentration-dependent growth in a basal salt medium utilizing PS powder (
Table 2) [
45]. Most importantly, a whole genome sequence and transcriptional analyses of
A. johnsoniii JNU01 uncovered an alkane-1-monooxygenase responsible for catalyzing PS hydroxylation.
3. Bio-Based Plastics
3.1. Polylactic Acid
While PLA is considered a biodegradable plastic, only 24 enzymes associated with PLA biodegradation have been extensively characterized until now [
72]. PLA undergoes degradation through enzymatic hydrolysis of its ester bonds, followed by the assimilation and metabolism of lactic acid oligomers, dimers, and monomers. PLA biodegradation is primarily facilitated by enzymes such as proteases, lipases, esterases, and cutinases, mainly found in genera such as
Amycolatopsis,
Alcanivorax,
Bacillus, and
Pseudomonas (
Table 1) [
30,
31,
73].
Numerous microorganisms have demonstrated the capability to degrade PLA, and one such strain is
Saccharothrix waywayandensis, which was isolated from soil. In a study, this microbial strain was reported to grow up to 37 mg of DCW when cultivated in a basal medium containing yeast extract and 100 mg of PLA film as the carbon sources (
Table 2) [
47]. In addition, this strain degraded 95% of PLA film within 7 days with the supplementation of 0.1% (
w/
v) gelatin, a well-known inducer of the hydrolases previously elucidated (
Table 2) [
74,
75]. The supplementation of gelatin proved to be highly effective in augmenting the expression levels of PLA-degrading hydrolases, thereby promoting a more efficient degradation process. Furthermore, during the course of PLA degradation, a transient increase in lactic acid generation was observed. Nonetheless, this temporary elevation in lactic acid levels was reduced as the strain assimilated the generated lactic acid.
3.2. Polybutylene Succinate
The degradation mechanism of PBS, along with PBS-degrading microorganisms and enzymes, is well established. PBS is known to undergo degradation through hydrolytic cleavage of the ester bonds, catalyzed by extracellular enzymes such as lipases and cutinases (
Table 1) [
32,
76]. These hydrolytic enzymes break down PBS into various components, including oligomers (bis(4-hydroxybutyl) succinic acid and 4-(4-((3-carboxypropanoyl)oxy)butoxy)-4-oxobutanoic acid), a dimer (4-(4-hydroxybutoxy)-4-oxobutanoic acid), and monomers (succinic acid and 1,4-BDO) [
77]. Furthermore, the oligomers and dimers resulting from PBS degradation are fully hydrolyzed into succinic acid and 1,4-BDO monomers, which then enter the central carbon metabolism.
Currently, PBS-degrading enzymes such as lipase Asahi from
Chromobacterium viscosum [
76] and lipase PS
® from
P. cepacia [
34] are commercially available (
Table 1). PBS-degrading microorganisms have predominantly been identified among fungi [
32] and microbial consortia [
78]. However, there is a need to explore other varieties of microorganisms to establish more efficient PBS degradation and bio-upcycling technologies. In a recent study, the
Bacillus sp. JY35 strain was isolated from wastewater sludge, demonstrating its ability to degrade a wide range of bioplastics, including PBS, PHA, and polycaprolactone [
79]. The whole genome sequencing of this strain revealed the presence of a carboxylesterase capable of degrading PBS. To explore the potential of this enzyme, an engineered
Escherichia coli strain was developed to overexpress the
Bacillus sp. JY35 carboxylesterase. The engineered
E. coli strain exhibited time-dependent PBS degradation, effectively breaking down 45.7% of 20 mg of PBS over a period of 10 days (
Table 2).
This entry is adapted from the peer-reviewed paper 10.3390/ijms242015181