Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Obesity, hypertension, insulin resistance, and dyslipidemia are all clusters of an entity called “Metabolic Syndrome”. The global trends of this syndrome’s incidence/prevalence continue to increase reciprocally, converting it into a massive epidemic problem in the medical community.
- atrial fibrillation
- metabolic syndrome
- obesity
1. Obesity
Atrial fibrillation is markedly more prevalent among obese individuals compared to those with a BMI below 30.0 kg/m2 [31,32]. Remarkably, even within the latter group, an elevated level of abdominal fat has been associated with an increased risk of AF occurrence [32]. Notably, an expanded body size during early adulthood, elevated BMI in midlife, and weight gain from the age of 20 to midlife have all been linked to an augmented likelihood of future AF development. This highlights that increased BMI at any stage of life can potentially serve as a risk factor for AF [33]. Individuals classified as obese face nearly a 50% heightened risk of developing AF when compared to their non-obese counterparts [34].
Obesity exerts a twofold influence by altering both cardiac hemodynamics and heart morphology. This leads to an amplified blood volume, increased cardiac output, remodeling of cardiac chambers, and an augmentation of epicardial fat [35]. Cardiac remodeling precipitated by obesity encompasses the emergence of left ventricular hypertrophy, resulting in diastolic dysfunction and pronounced enlargement of the left atrium [35,36]. These effects, in conjunction with the altered hemodynamics—such as heightened stroke volume and pulmonary pressures—establish an environment conducive to the initiation of AF [36]. The potential for echocardiographic left atrial volumetric enlargement over a decade is almost 2.4 times higher in obese individuals [37]. Moreover, pericardial adipose tissue significantly contributes to the burgeoning frequency of AF [38]. Of particular note, epicardial fat secretes adipocytokines, including Activin-1, which incites fibroblast proliferation and amplifies fibrosis within myocardial tissue [39]. This phenomenon plays a role in shaping the substrate required for AF.
Mahajan et al. highlighted the impact of obesity on cardiac tissue in sheep, demonstrating electroanatomical changes. Significantly reduced mean conduction velocity was observed in the obese group (LA 1.18 ± 0.04 m/s vs. 1.58 ± 0.04 m/s, p < 0.001) compared to the normal group. While the mean total voltage remained unchanged, distinct regional voltage patterns emerged in the left atrium of the obese and control groups, primarily due to a substantial reduction in posterior LA voltage (3.7 ± 2.3 mV vs. 5.5 ± 2.3 mV; p < 0.001) [40]. These findings were recently corroborated in men by the same research group [conduction velocity: 0.86 ± 0.31 m/s vs. 1.26 ± 0.29 m/s; p < 0.001]. In the obese group, 13.9% of all points in the left atrium exhibited low voltage, compared to 3.4% in the reference group (p < 0.001) [41]. These electrical changes contribute to the creation and maintenance of the substrate required for AF occurrence.
2. Hypertension
Hypertension contributes to around 14% of all cases of AF, and among AF patients, over 70% have hypertension. Hypertension independently amplifies the risk of AF progression and leads to the emergence of adverse effects associated with AF [42]. The impact of long-standing hypertension on the heart is well-documented, resulting in elevated cardiac filling pressures and diastolic dysfunction. A study from Norway established a link between diastolic dysfunction and AF [43]. Notably, this connection is applicable in cases with both left atrial enlargement and without it, where elevated left ventricular filling pressures primarily drive AF [44]. However, the most significant structural alteration attributed to hypertension is the enlargement of the left atrium (LA), influenced by various mechanisms encompassing hemodynamic and electrical remodeling, neurohormonal activation, and inflammation [45]. Electrical remodeling is also evident in individuals with hypertension, characterized by extensive areas of double potentials, fractionated signals, and varying and slower regional activation times within the atria, leading to atrial conduction delays [46]. Another noteworthy aspect of hypertension is the role of the renin-angiotensin-aldosterone system (RAAS), which becomes highly activated in hypertensive individuals. Experiments conducted on mice revealed that elevated levels of angiotensin II trigger both electrophysiological and structural modifications in the heart, along with increased fibrosis in the left atrium [47,48]. This fibrosis, as mentioned earlier, constitutes a significant factor in the development of AF [49]. Moreover, an alternate pathway through which hypertension may lead to fibrosis and subsequently to AF involves the extensive activation of the inflammatory process [50].
3. Insulin Resistance/Diabetes
Individuals with either type of diabetes have a twofold higher prevalence of AF, a figure that steadily rises as the severity of the disease and its microvascular complications progress. The presence of silent AF episodes is highly probable due to autonomic dysfunction [1]. Similar to hypertension, diabetes also involves structural and electrical remodeling, autonomic dysregulation, and inflammation. The renin–angiotensin–aldosterone system (RAAS) is enhanced, and elevated levels of angiotensin II are linked to diabetes, contributing to atrial fibrosis [51]. Another contributing factor in diabetes is the increased production of advanced glycation end products (AGEs) and their receptors, potentially exacerbating atrial scarring and fibrosis and thus contributing to the substrate for AF [52]. Consequently, patients develop diabetic cardiomyopathy characterized by diastolic dysfunction, which creates conditions within the atria conducive to AF occurrence [53]. Diabetic patients exhibit delayed conduction velocities and atrial emptying [54], intra-atrial electromechanical delay [55], prolonged action potentials, and abnormal atrial voltage [56]. These observations might be explained by the pathological expression of gap-junction proteins known as connexins [57]. Autonomic abnormalities, including sympathetic upregulation leading to sympathetic denervation and an imbalance in the autonomic nervous system—termed diabetic neuropathy—also impact the heart and can contribute to AF [58,59]. Inflammation and oxidative stress further contribute to the proarrhythmic state of diabetes. Disturbed mitochondrial function prevents the elimination of reactive oxygen species (ROS), leading to the activation of inflammation pathways evidenced by elevated inflammatory markers [60]. Additionally, hypoglycemia, with its accompanying sympathetic activation and fluctuations in blood glucose levels, can reinforce myocardial fibrosis and elevate oxidative stress, forming unique key mediators of the arrhythmic substrate in diabetes [61,62].
4. Dyslipidemia
The role of dyslipidemia in the occurrence of AF has not yet been fully elucidated. Low HDL cholesterol has been associated with an increased risk of AF, although elevated triglycerides have not shown a similar correlation [3]. However, findings from a Japanese cohort indicated that the relationship between low HDL and AF risk exists only in women, not men [63]. In contrast to the preceding clusters of MetS, the underlying mechanisms connecting dyslipidemia to AF have not been extensively investigated. Elevated blood lipids create an inflammatory environment and increase oxidative stress [64], potentially contributing to AF development. A comparison between patients with and without AF revealed that the AF group had 1.6-fold higher plasma triglycerides and increased inflammation markers [65]. Increased myocardial triglyceride content (MTGC), as measured by magnetic resonance spectroscopy, has been linked to diastolic dysfunction, although the precise connection between MTGC and plasma triglycerides remains unclear [66]. Recent studies have suggested a connection between postprandial, very low-density lipoprotein (VLDL), composed of triglycerides, and atrial remodeling in MetS patients [67]. Further support for this observation comes from new data demonstrating that a surplus of triglycerides within VLDL leads to atrial enlargement and disturbances in PR duration. In MetS patients, the left atrium diameter and volume were larger compared to non-MetS individuals (LA diameter: non-MetS 3.2 ± 0.3 cm vs. MetS-off statin 4.4 ± 0.4 cm vs. MetS-on statin 4.3 ± 0.3 cm, p < 0.0001; LA maximum volume: non-MetS 45.2 ± 9.5 mL vs. MetS-off statin 81.9 ± 13.9 mL vs. MetS-on statin 77.5 ± 18.9 mL, p < 0.0001; LA minimum volume: non-MetS 28.1 ± 7.5 mL vs. MetS-off statin 39.3 ± 10.8 mL vs. MetS-on statin 42.0 ± 11.3 mL, p = 0.0065). Additionally, atrioventricular conduction, as measured by PR interval, was prolonged in MetS patients (176.1 ± 19.0 ms vs. 156.2 ± 15.4 ms, p = 0.0014) [68]. Therefore, it is plausible that dyslipidemia contributes to structural and electrical changes in the cardiac chambers, yet further research is warranted in this area.
5. Metabolic Syndrome
It is readily apparent that when these risk factors converge within the framework of “Metabolic syndrome,” they profoundly disrupt the proper functioning of the left atrium, precipitating the occurrence of AF through a multitude of mechanisms. Overall, there is insufficient evidence to substantiate the direct association of MetS as a distinct entity with AF. Instead, much of the research has focused on the individual components of MetS in relation to AF. A rat study revealed that obesity compounds the atrial arrhythmogenic phenotype in hypertensive rats, exacerbating interstitial atrial fibrotic changes, conduction velocities, and left atrial emptying [69]. This lends credence to the idea that MetS, as an integrated entity, can indeed instigate AF. Additionally, evidence suggests that MetS patients experience both structural alterations—such as left ventricular and left atrial remodeling—and electrical changes (PR interval in MetS group vs. non-MetS: 167.6 ± 20.0 msec vs. 156.2 ± 24.9 msec, p = 0.0064) [67,68]. Furthermore, both MetS and AF are characterized by inflammation, and their coexistence has been associated with even greater levels of inflammation, potentially introducing another mechanism that leads to fibrosis and AF development [70]. Beyond inflammatory markers, other biomarkers dysregulated in MetS—such as adiponectin, leptin, ghrelin, uric acid, and OxLDL—appear to contribute to the initiation and progression of AF. Adiponectin appears to mitigate cardiac chamber remodeling [71]. Leptin-mediated pathways impact Angiotensin metabolism, thus contributing to AF [72]. Moreover, since leptin regulates calcium homeostasis, it significantly influences electrophysiological pathways [73]. Lastly, MetS disrupts autonomic tone, with the degree of impairment corresponding to the number of MetS clusters [74]. This observation suggests that MetS could potentially play a role not only in the formation of substrate but also in triggering AF (Figure 2).
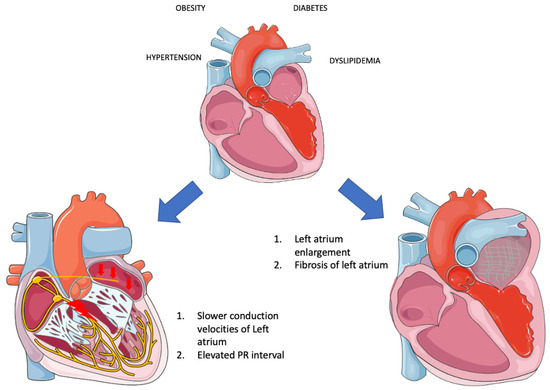
Figure 2. Electrical and structural remodeling in metabolic syndrome (Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed on 20 August 2023).
The human heart, alongside skeletal muscles, kidneys, and the brain, is a highly energy-consuming organ, necessitating significant amounts of adenosine triphosphate (ATP) molecules for proper function. Approximately 60% of the required energy is derived from fatty acid (FA) metabolism through β-oxidation [75]. However, this process is accompanied by a reduction in the NADH/NAD+ system within mitochondria, leading to the generation of electrons that enter the electron transport chain (ETC) to produce ATP. In situations where β-oxidation becomes overactive, or ATP levels decrease, an excess of electrons is generated, resulting in the conversion of these electrons into superoxide radicals (ROS) [76]. Metabolic syndrome has been demonstrated to lead to a condition in which the heart predominantly relies on FA for ATP production, imposing excessive stress on mitochondria and causing damage through various mechanisms [77]. The malfunctioning ATP production mechanisms lead to a decreased ATP-to-O2 consumption ratio, triggering hyperactivity of the cardiac muscle, subsequent hypertrophy, and diastolic dysfunction. Moreover, mitochondria play a crucial role in maintaining Ca2+ homeostasis. Thus, mitochondrial dysfunction results in disruptions and oscillations of intracellular Ca2+ levels, potentially inducing arrhythmias [78]. Lastly, due to the incapacity of mitochondria to generate ATP and their shift towards ROS production in the ETC, substantial quantities of ROS are released into circulation [77]. As mentioned earlier, ROS triggers inflammation, leading to structural remodeling of the heart as previously described. Consequently, recent findings underscore the significance of mitophagy—the process that selectively eliminates damaged mitochondria from cells, facilitating their degradation by lysosomes and maintaining mitochondrial homeostasis—in the pathophysiology of Metabolic Syndrome (MetS). There is a suggestion for its potential therapeutic application in the future [79].
This entry is adapted from the peer-reviewed paper 10.3390/jpm13091323
This entry is offline, you can click here to edit this entry!
