Cellulose is the most abundant natural polymer on Earth. The roles of cellulose nanocrystals’ (CNC) nonlinear geometry in improved performance coatings are appealing for mini structures or device production; yet, CNC is an insulating material. The cellulose nanocrystal (CNC) is a part of the organic crystallization macromolecular compound found in plant fibers and bacteria’s capsular polysaccharides. It has several properties, which include high strength, high crystallization, large physical properties, minimum density, and good biocompatibility. CNCs, with their exceptional mechanical, thermal, and optical properties, have emerged as a versatile and sustainable nanomaterial with the potential to revolutionize various industries.
1. Introduction
CNC has a minimal density, specific impact resistance, low thermal expansion, superior stiffness, elongated geometry, barrier properties, an elevated surface area and relative density, and bio-conjugation availability and simplicity. Because of the numerous reactive chemical groups on its surface, chemical vapor deposition, physical adsorption, and surface grafting can all be used to enhance its performance. CNC research has received much interest in the last few years, as seen by the significant rise in academic papers and patents published worldwide. As a result, it is extensively applied in industries including construction, cuisine, telecommunications, medication, and barriers
[1]. Carbon nanotubes (CNTs) are another rod-shaped nanomaterial with excellent mechanical and electrical conductivity performance.
Cellulose, when combined with the functionality of reduced graphene oxide, has unique advantages. Kafy and his associates concluded that graphene oxide improves the electronic communication between enzymes
[2]. Graphene oxide measures the alterations in the electric conductivity of the material. Graphene also possesses unique surface features, such as the ability to modify its surface. The advantage of graphene is that it is a very low-noise material. As a result, graphene’s carrier concentration can alter dramatically even with no carriers and a few more electrons. In this application, graphene also enables the generation of four-probe devices on monocrystals, which is an added benefit. This ensures that the contact resistance’s role in limiting sensitivity is no longer a factor
[3].
Nanocellulose has been used to enhance materials’ physical and dispersibility attributes because of its high stiffness, special water dissolution rate, and hydrophilic aspects. Hydrolyzed polymeric cellulose surface morphology reduces obstacles to more significant movement between the analyte and the immobilized indicator, resulting in a faster response time
[4]. The cellulose nanocrystal film is an attractive fit for wearable sensors because of the high energy engagement between the binding site on the nanocrystalline cellulose film and the charged species and the membrane separation properties
[5]. CNC stands out among other nanostructured materials as a biocompatible, nontoxic, sustainable, and renewable nanomaterial. CNC has numerous potential uses in multiple fields, for instance, materials science, electronics, medicine, and many more, owing to its nanometric size, huge aspect ratio, and superior chemical and mechanical characteristics.
Cellulose is abundant in plants and microbes and significantly impacts the synthesis of macrostructure polymer materials. Cellulose nanocrystal has shown their importance in various uses in the industry due to their excellent material characteristics
[1]. Cellulose nanocrystals are distinct nanoparticles generated from the most prevalent and seemingly natural polymer, inexhaustible cellulose. These nanostructures’ chemical, rheological, optical, and mechanical properties have attracted people’s interest. Cellulose nanocrystals, often generated from pre-existing cellulose fibers, are biodegradable and renewable, making them ideal for various uses
[6]. Although naturally hydrophobic, these nanocrystals can be surface-functionalized to meet multiple demanding requirements, including creating high-performance nanocomposites with hydrophobic polymer matrixes
[7]. This research attempts to consolidate knowledge concerning cellulose nanocrystal sources, chemical composition, chemical isolation procedures, and physical and rheological, mechanical, and optical properties considering the growing interdisciplinary research being conducted on them. Innovative applications of cellulose nanocrystals in domains as diverse as bioengineering, material sciences, semiconductors, catalysis, and various others are emphasized
[8].
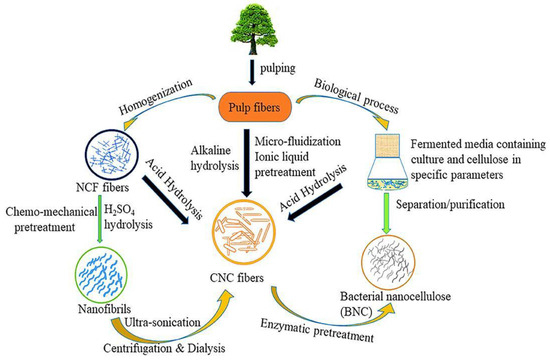
The preparation processes (
Figure 1) of the CNC were evaluated in this research as sustainable future material. Meanwhile, brief introductions were given to CNC’s significant applications in composites, energy consumption, semiconductors, and barrier film. The comprehensive preparations and applications yielded practical concepts and methodologies for future high-end and environmentally friendly functional composites. There were also some suggestions for extended probable applications
[9].
Figure 1. Production process of nanocellulose
[10].
Plant fibers are commonly used in preparing CNCs due to their inexpensive cost and abundance, with a standard length and diameter. Meanwhile, the CNC refers to the size of any one-dimensional cellulose located in the range of 100 nm
[11]. As a result, the processes for obtaining the CNC reduce the size of natural cellulose, which include mechanical, chemical, biological, and combination.
2. Mechanical Process of Preparation of CNC
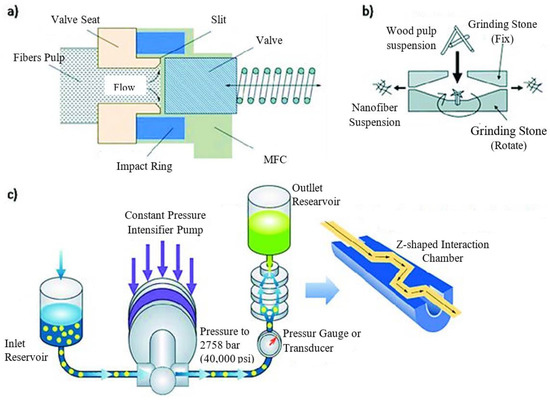
The mechanical process (Figure 2) includes four steps and is a physical approach to obtaining the CNC:
Figure 2. Operation schemes of (
a) high-pressure homogenizer; (
b) micro grinder from Masuko Sangyo Co.; and (
c) micro fluidizer from Microfluidics Inc.
[12].
- (i)
-
Homogenization under high pressure;
- (ii)
-
Micro fluidization;
- (iii)
-
Fine grinding;
- (iv)
-
Freezing smashing.
2.1. Homogenization under High Pressure
It takes just one step to homogenize a solid into ultrafine particles in a solution, which results in a stable suspension. High-pressure homogenization was the most common method for producing microcrystalline cellulose (MCC). Several investigations have shown that it can be used to make MCC from various basic materials
[12].
2.2. Micro Fluidization
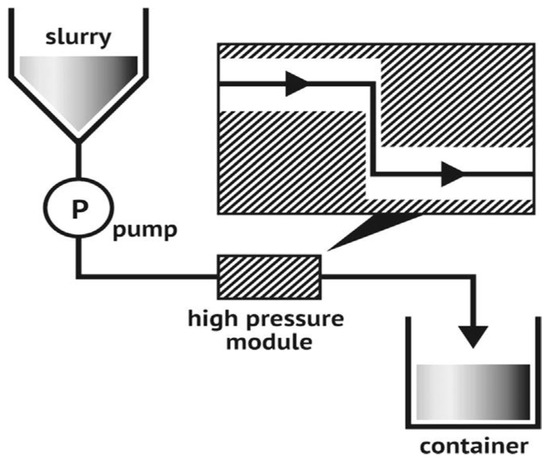
Figure 3 depicts the technical process of micro fluidization under high pressure, one way of creating nanomaterial. The fluid pump initially injected and compressed the raw slurry to a pressure of roughly 4000 bars. After that, it was thrown into a Y-shaped interactive tank, where two liquid squirts traveling at 1000 m/s collided. The vacuum effect and enormous shear force generated by this impact lead to the production of nanoparticles
[13].
Figure 3. A schematic diagram of the micro fluidization process
[14].
2.3. Fine Grinding
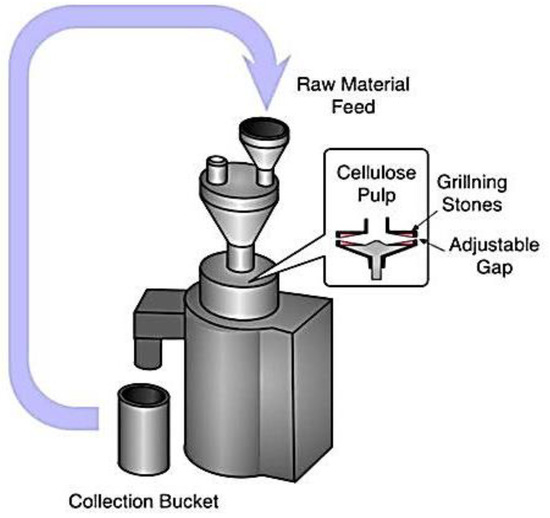
The fine grinder and a commercial grinder worked together to provide a new physical approach to making MCC. It was clear that the main components were made up of two discs, one internal and one exterior, each covered with grooves with a unique set of characteristics. As the two discs rotated in tandem, they applied crushing, shearing, friction, grinding, and ripping forces that caused the fibers to split and shrink. However, it was very inefficient, and only a few instances of its use were documented
[13]. In
Figure 4, the experimental setup for fine grinding can be seen. The raw material is poured into the funnel, and, inside the chamber, the cellulose pulp passes through grilling stones and an adjustable gap. Finally, the finished product is collected from the collection bucket.
Figure 4. Ultra-friction grinder for the synthesis of cellulose nanofiber (CNF)
[13].
3. Chemical Process
The CNC can only be obtained by partly disrupting glycosidic bonds; chemical hydrolysis makes a strong argument for attaining this. A kind of polysaccharide, cellulose, is created from glucose molecules joined together by the β-1,4-glucosidic link
[15].
3.1. Alkali Hydrolysis
Natural cellulose’s crystal shape changed from I to II after being subjected to a 9-weight-percent NaOH treatment. The alkali has the power to both expand the cellulose and rupture its internal hydrogen bonds. Even though alkali has been used to hydrolyze cellulose in several studies, alkali was mainly used to dissolve lignin and pectin
[16].
3.2. Acid Hydrolysis
Repeating the above structures yielded the cellulose fiber, composed of discontinuous sections of crystalline and amorphous cellulose. The CNC was made from reed pulp utilizing sodium m-nitrobenzene sulfonate (SMS) as a cocatalyst and 55-weight-percent sulfuric acid. The final CNC had an I-form and a rod-like shape, measuring 12 nm in diameter and 146 nm in length. Its crystallinity was 76.1 percent. On the other hand, the CNC-produced thermal insulation foam demonstrated its flawless thermal insulation ability under typical conditions. The ideal time range for the hydrolysis of this fiber was discovered to be between 45 and 55 min
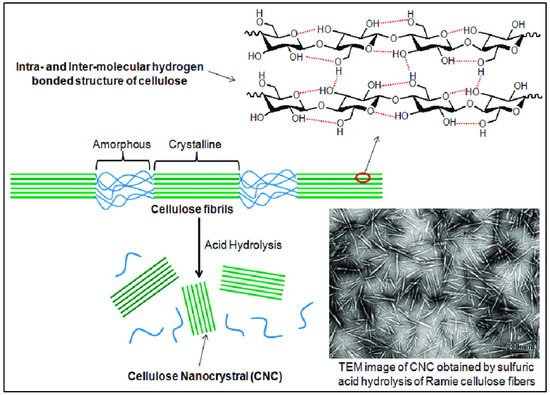
[17]. the internal structure of cellulose can be seen (
Figure 5), where two types of regions are present. An unorganized blue portion is called the amorphous region, and the green liner pattern is called a crystalline region. To obtain CNC from the amorphous cellulose region, concentrated 98% sulfuric acid must be broken.
Figure 5. Manufacturing of CNC by acid hydrolysis and the corresponding TEM image
[18].
This entry is adapted from the peer-reviewed paper 10.3390/polym15204070