Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pediatrics
Folates refer to a class of B9 vitamers with a basic structure composed of heterocyclic pteridine moiety covalently linked via a C9-N10 methylene bridge to p-aminobenzoylglutamate. Auxotrophic primates like human beings rely on exogenous dietary vitamin B9 supplementation to meet their metabolic demands. Folates play a crucial role in nucleotide synthesis and DNA methylation. Maternal folate deficiency causes several pregnancy-related complications, perinatal defects, and early childhood cognitive impairments.
- folic acid
- NTDs
- hypertension
- pre-term birth
- stillbirth
- spontaneous abortion
- cognition
- GDM
1. Introduction
Deoxyribonucleic acid (DNA), the genetic biopolymer repository synthesised and conserved within eukaryotic cells, is composed of deoxynucleotide triphosphate (dNTP) monomers. Deoxythymidine triphosphate (dTTP), a key dNTP, is synthesised by thymidylate synthase in the presence of folate derivative as cofactor. During pregnancy, the exponential increase in nucleotide biosynthesis supports rapid cell proliferation and tissue formation in the developing foetus. It creates a high metabolic demand for essential nutrients, usually quenched through maternal dietary intake or supplementation. Vitamin B9 or M (IUPAC name: (2S)-2-[[4-[(2-amino-4-oxo-3H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid) is essential for nucleotide biosynthesis, DNA methylation, and amino acid homeostasis in cells [1]. Folates refer to a class of B9 vitamers with a basic structure composed of heterocyclic pteridine moiety covalently linked via a C9-N10 methylene bridge to p-aminobenzoylglutamate (Figure 1) [2]. The biological activity and bioavailability of B9 vitamers vary with oxidation state, substitutions, and the number of glutamate chains. Since humans lack the molecular machinery for de novo folate cofactor synthesis, the exogenous ingestion of natural folate polyglutamates and synthetic folic acid (FA) vitamer supplements support the nutritional demand. The ingested folate polyglutamates hydrolysed to monoglutamates, are converted to 5-methyltetrahydrofolate in the intestinal mucosa prior to entering circulation [3]. This circulated folate is then converted to 7,8-dihydrofolate (DHF) and 5,6,7,8-tetrahydrofolate (THF) by the cells to meet the metabolic demand [4,5].
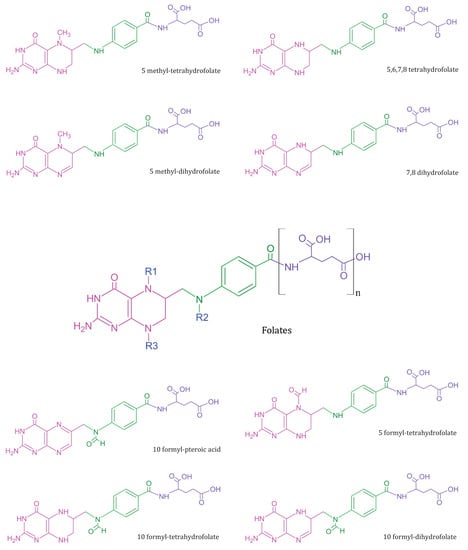
Figure 1. Summary of common folate vitamers—Folates (in centre) represent a general template structure, composed of a pteridine ring (in pink), p-aminobenzoic acid (in green), and glutamic acid moiety (in purple). Most metabolically active B9 vitamers exist in monoglutamate form and vary in structure based on R1, R2, and R3 substitutions.
The stability of B9 vitamers is reviewed elsewhere [6]. The normal range of folates is about 5–15 ng/mL in serum, 16–21 ng/mL in cerebrospinal fluid, and 175–316 ng/mL in erythrocytes [7]. To maintain the normal folate range, the daily recommended folate intake is 400 µg folate per day for adults [8]. Serum folate levels below the normal range indicate a deficiency condition. Multiple lines of evidence implicate folate deficiency in a variety of conditions during pregnancy that severely impact maternal and child health. Among such conditions, neural tube defects, pre-eclampsia, pre-term birth, stillbirth, spontaneous abortion, cognitive impairment, childhood cancers, polycystic ovary syndrome, and postpartum maternal mental health contribute substantially to the global health burden [9,10,11,12].
Among pregnant women who received at least 400 µg FA daily through fortified food and vitamin supplementations, complications such as neural tube defects dropped nearly by 50%. In women with a history of neural tube defects (high risk pregnancies), the daily dose of FA is recommended 1–3 months prior to conception [13]. Based on such well-substantiated evidence, strong folic acid fortification frameworks and policies are taking shape to tackle folate deficiency globally [14,15,16]. On the other hand, recent evidence suggests excess FA intake could also be detrimental to health [17].
Together, the danger of folate deficiency and excess highlights the need to monitor appropriate FA intake, especially during preconception and perinatal periods. At present, maternal folate levels are measured in clinical or laboratory settings. Intriguingly, an increase in stillbirth and neonatal mortality rates was observed across different countries during the COVID-19 pandemic [18,19]. This observation highlights the impact of dependency on clinical infrastructure for perinatal healthcare. Although unexpected, the need for point-of-care devices to bring quality perinatal healthcare to communities was evident during the pandemic.
Folates have been implicated in maternal and perinatal health for at least three decades. The role of various folates in metabolic homeostasis are summarised elsewhere [20,21,22]. In the following section, the focus is on the associations between maternal folate deficiency and different perinatal complications.
2. Neural Tube Defects
The World Health Organization (WHO) reports an annual global estimate of 240,000 neonatal deaths within the first 28 days due to congenital disorders as of 2023. Neural tube defects (NTDs) occurring in weeks 3 or 4 of pregnancy are among the most common and extremely severe but preventable congenital disorders [23]. Anencephaly, the most fatal of NTDs, is a congenital brain malformation, where the anterior (cranial) neural arch fails to close during embryo development. This condition invariably results in stillbirth or neonatal mortality [11,24].
While anencephaly occurs early in embryogenesis, the same malformation occurring late in embryogenesis results in encephalocele. Typical brain-tissue protrusion through the skull, mostly in the occipital region, is a characteristic of encephalocele. It is relatively rare compared to anencephaly, yet presents a high mortality risk [25]. Another common NTD, spina bifida (SB), is a congenital spinal cord malformation, wherein the posterior (caudal) vertebral arch fails to close, resulting in meningocele, myelomeningocele, and/or hydrocephalus [11,26]. In the case of SB, though the mortality rate is relatively lower than anencephaly, the patients are likely to experience neurological disabilities.
Within the proposed multifactorial aetiology of NTDs, nutritional deficiency arguably is the most easily preventable factor [27,28]. FA supplements and fortification were reported to reduce NTDs related to neonatal mortality [11,28,29,30]. In particular, two exemplary large-population, data-based analyses concluded periconceptual maternal FA intake prevents NTD occurrences [31,32]. Another meta-analysis of NTDs in eastern Africa also suggests that mandatory FA supplements could reduce the risk of NTDs [33]. Fascinatingly, an 11-year follow-up study by Caffrey et al. shows that continued FA supplementation post the timeframe recommended to prevent NTDs supports neurocognitive development in the baby [34].
3. Hypertensive Disorders
Pregnancy-induced hypertension (PIH) reduces nutrition and oxygen supply to the foetus. PIH includes chronic hypertension, pre-eclampsia (PE), superimposed pre-eclampsia, and gestational hypertension [35]. PE clinically manifests as hypertension and proteinuria occurring post-week 20 of gestation [36]. The aetiology of PE remains largely unknown. Moreover, unlike other pregnancy-related complications, PE is detrimental to both maternal and foetal health [37]. Strikingly, a key observation in PE and gestational hypertension is elevated levels of homocysteine in blood [12]. It is well-established that FA supplements mitigate the risk posed by elevated homocysteine levels [38].
Not surprisingly, controversial evidence for nutritional supplementation in reducing PE risk does exist [39,40]. Yet, the overwhelming amount of evidence highlights FA supplementation as a protective factor against PE [12,36,41,42,43,44].
4. Pre-Term Birth
The WHO estimated around 13.4 million pre-term births (PTB) in 2020, which is a leading cause of neonatal mortality. For human beings, 40 weeks marks the normal gestation period. Any spontaneous pre-term labour between the 28th and 37th week of gestation is termed PTB [45]. The complications associated with PTB are diverse and include cerebral palsy, hypothermia, hypoglycaemia, brain injury, and cognitive impairment [46]. Tackling PTB is unquestionably a global priority. In May 2023, the United Nations International Children’s Emergency Fund (UNICEF) published an updated report on PTB, titled “Born Too Soon: Decade of action on preterm birth”. This report found that preterm births have not changed significantly in any region of the world in the past decade. Southern Asia and Sub-Saharan Africa account for more than 65% of preterm births recorded globally. In Singapore, nearly 9% of pregnancies end in PTB, and the trend is expected to increase in the near future [47].
Furthermore, this report proposes an action plan and identifies intersectoral risk factors influencing PTB, among which maternal nutrition is of high significance. Notably, a retrospective cohort study including 200,000 women observed a reduced PTB risk with FA supplementation [46]. Another case-controlled study reported low maternal serum folate levels in PTB cases [45].
A recent meta-analysis of epidemiological studies correlating PTB risk and maternal folate levels also conclusively states that PTB risk reduces with higher maternal folate levels [48]. Lastly, an observational study observing the high maternal folate level association with low PTB risk even proposed possible maternal folate level optimisation in the post-fortification era [49].
5. Stillbirth and Spontaneous Abortion
In 2014, 194 countries endorsed the “Every Newborn Action Plan (ENAP)” developed by the WHO. The ENAP sets a global target to achieve less than 12 stillbirths per 1000 total births in all 194 countries by 2030. Stillbirth is the loss of the foetus post week 20 of gestation. Approximately 1.9 million babies, or one every 16 s were born stillborn in 2021. The major causes of stillbirths include placental malperfusion, foetal asphyxia, congenital malformations, infection, PTB, PE, and umbilical cord complications [50,51,52]. Spontaneous abortion (SA) refers to the loss of a foetus prior to week 20 of gestation [53]. Several factors such as chromosomal abnormalities, nutritional deficiency, immunogenic factors, DNA fragmentations, lifestyle choices, and other factors could result in SA [53,54]. In accordance with such findings, studies suggest periconceptional FA supplementation reduces the risk of SA and stillbirths [55,56,57,58,59].
6. Early Childhood Cognition
Cognition, occasionally dubbed the ultimate brain function, develops extensively during early childhood. Based on accumulating evidence highlighting the role of periconceptional FA supplementation in foetal neurodevelopment, studies hypothesised its influence on cognition and motor abilities. Fascinatingly, a study of children aged 4–5 years reported that low maternal FA levels resulted in attentional dysfunction preferentially among boys [60]. A longitudinal study of children aged 7–9 years, from the same group, reported that low maternal FA results in low alertness irrespective of gender.
Interestingly, they also observed comparatively better cognitive and working memory among girls [61]. Another study conducted in Japan reports a higher cognitive, language, and social development quotient among 4-years-olds whose mothers began FA supplementation before week 12 of gestation [8]. Although such strong correlations are well-substantiated, certain previous studies providing insignificant correlations do exist [62]. This might be due to several postnatal factors influencing infant development through early childhood.
7. Genomic and Epigenomic Instability
Most intriguingly, the latest sets of evidence hint at the need to study the optimal threshold for FA intake. As summarised above, low FA intake results in adverse pregnancy outcomes. On the other hand, excess FA supplement consumption could induce genomic and epigenomic instability [63,64]. Although further studies on the human population is essential to estimate the true impact of high and low FA intake, it is highly likely that excess FA intake could also result in unfavourable outcomes.
8. Gestational Diabetes Mellitus
Among different pregnancy-related complications, the most common metabolomic disorder is gestational diabetes mellitus (GDM) [65]. GDM refers to glucose intolerance occurring in early pregnancy and is estimated to affect 30% of pregnancies globally [66]. In Singapore, GDM is the most prevalent metabolomic pregnancy-related disorder, observed in roughly 25% of pregnancies. Several studies have reported a strong association between GDM and high FA with vitamin B12 insufficiency [67,68,69]. Although the aetiology of GDM remains elusive, current observations consistently report the imbalance in FA and vitamin B12 concentrations, postulating a potential role for surplus FA in GDM.
This entry is adapted from the peer-reviewed paper 10.3390/bios13100912
This entry is offline, you can click here to edit this entry!
