Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biophysics
|
Cardiac & Cardiovascular Systems
Cardiac arrhythmias are a major cause of cardiovascular mortality worldwide. Many arrhythmias are caused by reentry, a phenomenon where excitation waves circulate in the heart. Optical mapping techniques have revealed the role of reentry in arrhythmia initiation and fibrillation transition, but the underlying biophysical mechanisms are still difficult to investigate in intact hearts.
- cardiac tissue
- cardiomyocytes
- ion currents
- excitation wave
- ips cells
1. Introduction
The cardiovascular system relies on two physiological systems: the myocardial contractility and the electrical excitation conduction through the cardiac tissue. The myocardial contractility enables blood pumping, while the electrical excitation ensures synchronized heart muscle contraction. Most cardiovascular arrhythmias result from disturbances in excitation conduction, leading to uncoordinated muscle fiber contraction. These disturbances are studied in electrophysiological experiments on heart preparations, which are either whole isolated perfused hearts or separate cardiac tissue slices. The main limitation of these studies is that the heart preparations gradually deteriorate, and their long-term survival, which guarantees the stability of physiological processes in the preparations, is unfeasible. In the last two decades, new approaches to studying arrhythmias in cardiac tissue culture models have been developed. These approaches have allowed a deeper understanding of arrhythmia mechanisms and new methods for their treatment.
Tissue models are in vitro multicellular systems that consist of cardiac cells arranged in two- or three-dimensional structures. They can be derived from primary cardiac cells (from animals), pluripotent stem cell-derived cardiac cells, or immortalized cardiac cell lines. Tissue models have several advantages over conventional single-cell models or animal models for studying cardiac arrhythmias [1,2]. They can
-
Explore the mechanisms of arrhythmias in detail by isolating and examining individual cells or small cell clusters;
-
Evaluate new drugs and treatments for arrhythmias by simulating the conditions in the heart and monitoring how they affect the heart’s electrical activity;
-
Investigate the effects of different environmental factors on arrhythmias by exposing cells to various chemicals, toxins, and other environmental factors and measuring how they affect the heart’s electrical activity.
In summary, tissue culture models can recreate the structural and functional heterogeneity of the human heart tissue, as well as allow for precise control and manipulation of the cellular and extracellular environment. They enable high-throughput screening of drugs and gene therapies, and they reduce the ethical and practical issues associated with animal experiments.
Tissue models have been used to investigate various types of cardiac arrhythmias, such as atrial fibrillation, ventricular tachycardia, and catecholaminergic polymorphic ventricular tachycardia (CPVT). For example, researchers have created tissue models of CPVT, a rare inherited arrhythmia that can cause sudden cardiac death in young people [3]. They used induced pluripotent stem cells (iPSCs) from patients with CPVT to generate cardiac cells that exhibited abnormal calcium handling and spontaneous arrhythmias [4]. They then used these cells to create two-dimensional monolayers or three-dimensional engineered heart tissues that replicated the patients’ arrhythmias in vitro. Furthermore, they tested a gene therapy approach that corrected the underlying mutation and restored normal calcium handling and rhythm in a mouse model of CPVT3 [5].
However, while tissue models are promising tools for advancing our knowledge of cardiac arrhythmogenesis and developing novel therapeutic strategies, they also face some challenges and limitations, such as the following:
-
The challenge of replicating the exact cellular composition and architecture of the human heart tissue;
-
The variability and immaturity of iPSC-derived cardiac cells compared to native cardiac cells;
-
The lack of standardized protocols and criteria for producing and assessing tissue models;
-
The need for further validation and translation of tissue model findings to human clinical settings.
Therefore, future research should aim to improve the quality and reproducibility of tissue models, combine them with other technologies such as tissue engineering, gene editing, optogenetics, and computational modeling, and establish their relevance and applicability for human cardiac arrhythmia research and therapy. To create a biomimetic of cardiac tissue, several parameters of the system could be varied (Figure 1):
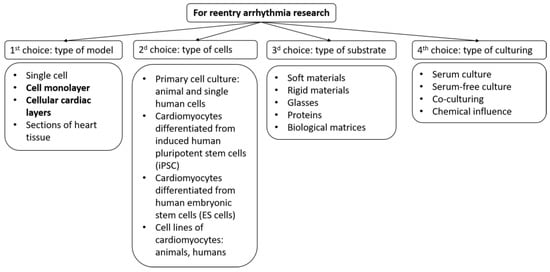
Figure 1. Selection scheme for creating a model of cardiac tissue to study arrhythmias. The figure highlights those models on which this review focuses.
-
Cell type;
-
Type of substrates and coatings for cell seeding;
-
Type of growth medium and external factors.
An important role in the creation of artificial tissue is played by the purpose of its creation. In this review, we focus on testing the arrhythmogenicity of the tissue itself or drugs. But even in such cases, for a complete picture of arrhythmogenicity, it is necessary to vary the conditions under which testing is performed. The following are two main variations:
-
Simulation of a conduction case in real heart tissue: fibrosis, anisotropy, domain location, and more;
-
Congenital mutations leading to arrhythmias.
2. Cardiac Tissue Models
Cardiac arrhythmia refers to any deviations from the normal electromechanical functioning of the heart, which involves disturbances in the generation and propagation of a cardiac impulse [6]. The main disturbances in impulse propagation result from various conduction blockades and reentrant rhythms, which occur when a cardiac impulse re-excites a certain area of the myocardium under certain conditions. Re-entry causes the most dangerous cardiac arrhythmias, such as ventricular tachycardia and atrial and ventricular fibrillation, which may be associated with spiral waves and may occur after heart attacks or strokes [7,8]. Re-entry can be either ordered when the impulse travels along a fixed anatomical path or random when the path is constantly changing [9]. Fibrillation is a phenomenon of multiple random re-entry circuits in an excitable medium such as cardiac tissue. The conditions for maintaining re-entry in an excitable medium include prolonging the conduction time or shortening the effective refractory period [10]. Spiral waves have also been observed in various autocatalytic types of chemical reactions, such as the Belousov–Zhabotinsky reaction, and biological systems [10,11,12]. When the ratio of speed, refractory period, and obstacle radius is certain, a wave can circulate an element of the medium located at the edge of the obstacle several times [13,14]. Re-entry as a fundamental reason for arrhythmias can also be studied in simulated cardiac tissue models (Figure 2).
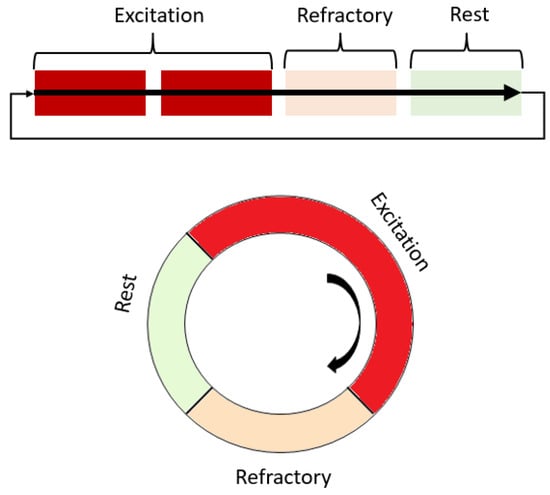
Figure 2. Scheme of the operation of the excitation and spiral wave according to the states that a single element of the excitable medium passes through. Red shades highlight areas that are non-excitable during work or partially excitable.
Rotating waves, or reentries, are a type of abnormal electrical activity in the heart that can lead to many life-threatening cardiac tachyarrhythmias. Antitachycardia or overdrive pacing (ATP) is a technique that delivers rapid electrical stimuli to the heart to restore normal rhythm [15,16,17]. However, the exact mechanism of how ATP works is not fully understood and remains an active area of research. This is because studying this phenomenon poses two main challenges. First, it is hard to predict how electrical waves propagate in a real heart, especially when it is affected by diseases that make it more complex and heterogeneous. Second, it is technically difficult to record the propagation patterns in a real heart with high enough temporal and spatial resolution to reveal the fine details of how rotating waves and paced wave fronts interact. Therefore, most of our knowledge about the mechanisms of reentry termination by an external source initially comes from numerical studies [18].
Using simplified models of cardiac tissue, such as thin layers of cardiac cells grown in culture, we were able to gain some insights into the mechanism of ATP. These studies have examined how pacing affects reentrant activity and have provided insights into the motion of a spiral tip, which is the point where the rotating wave curls around itself. The motion of the spiral tip is relevant to the idea of paced-induced spiral drift, which is a potential mechanism of ATP that involves moving the spiral away from its original location by pacing. In most cases (about 80%), we successfully terminated functional reentry by overdrive pacing [19,20]. We used confocal microscopy to record images with high spatiotemporal resolution and track the trajectory of a spiral wave tip in an interactive mode [21]. We visualized how a spiral wave tip interacted with the wavefront of paced waves in cardiomyocyte cultures. Our data suggest that (1) stable spiral waves in cardiac monolayers tend to be pinned to local microheterogeneity, which are small areas with different electrical properties than their surroundings; (2) overdrive pacing can shift a rotating wave from its original site by colliding with its tip and causing a wave break; and (3) the wave break, formed as a result of the interaction between the spiral tip and the paced wavefront, can be moved by a pace-induced drift mechanism to an area where it becomes unstable or collides with a boundary and terminates.
The above experiments with cultured cardiac myocytes have suggested that one possible mechanism of spiral wave termination by ATP is the induced drift of the rotating wave and collision with the tissue boundary [22,23]. However, this mechanism may not apply to pathological rotating waves in the heart tissue, which are not “free spirals” but rather “pinned spirals”. Due to the heterogeneity of the heart tissue, spiral waves tend to attach to local variations in tissue excitability and become stabilized as pinned rotating waves [24,25]. Tung and co-workers showed that rotating spirals can spontaneously pin to relatively small obstacles in cardiac myocytes [26]. Therefore, unpinning rotating waves from anatomical obstacles is a key step for the success of ATP.
Excitation waves have a minimum velocity to propagate successfully [27]. Propagation failure may occur when the wavefront velocity near the obstacle drops below this critical value [28]. Two factors can reduce the wavefront velocity: increased front curvature and high-frequency pacing. The first factor is that the wave velocity depends on the front curvature in two-dimensional excitable media [25,29]. When a paced wave attaches to an obstacle, it deforms and slows down because of the curvature effect. Moreover, the front curvature increases closer to the center of a spiral wave. Since the obstacle acts as a core for the pinned spiral wave, the wavefronts near the obstacle are always slower and reach the critical minimum velocity faster than the outer wavefronts. Thus, the wave fails to propagate close to the obstacle under a sufficient pacing rate, which results in a detachment or “unpinning” of the wave. After that, the spiral wave drifts according to the described mechanisms [30]. As for the pacing frequency for unpinning and successful ATP, it must be higher than a critical one. The frequency window for successful ATP of pinned rotating waves is narrower than for the case of free rotating waves, and it is more narrow for larger radii of the obstacle. For unpinning and successful ATP, the pacing frequency must be higher than a critical one. The frequency window for the successful ATP of pinned rotating waves is narrower than for free rotating waves, and it narrows further for larger obstacle radii.
In addition to ATP, the problem of spiral wave unpinning can also be solved by various non-invasive methods, which also make it possible in the future to eliminate reentry as the cause of certain types of arrhythmias. These methods are usually based on controlling cardiac excitability on the sensitivity of cells to light and include optogenetic approaches and photocontrol mediated by photosensitive substances. In particular, synthetic photosensitive molecules are used that are capable of photoisomerization upon absorption of photons. For example, such substances are azobenzene and its derivatives [31]. In our works, we tried to determine how effective the photocontrol method can be for eliminating arrhythmias [32,33]. We investigated the effect of a simulated azobenzene trimethylammonium bromide excitability gradient in cultured heart cells on the behavior and termination of reentry waves. Experimental data indicate a shift of the reentry wave mainly towards lower excitability.
In addition to approaches that simulate various excitability of cardiac tissue using substances, approaches based on genetic changes exist. The following study [34] shows that spiral waves in the monolayers of atrial cardiomyocytes can be effectively terminated using a light-induced depolarizing current generated by the arrhythmogenic substrate itself with optogenetic engineering. This result was shown in experiments using neonatal rat cardiac tissue by vector insertion of light-dependent rhodopsin into the tissue, directly affecting the dynamics of calcium.
Another study proposed a fundamentally new phenomenological concept that involves artificially dragging spiral waves by their cores to prove that manipulating the core of a spiral wave should lead to complete spatiotemporal control over its dynamics. The study was performed with in vitro testing on optogenetically modified monolayers of rat atrial cardiomyocytes [35]. As a result, it was possible to achieve reentry termination in several cases compared to the control, which confirms the concept of shockless defibrillation.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics8060487
This entry is offline, you can click here to edit this entry!
