Significant research efforts have been focused on the catalytic transformation of glycerol for the synthesis of value-added chemicals owing to the rising prices of petroleum resources. Glycerol is an important byproduct due to its application to produce acrolein, glyceric acid, glycerol carbonate, and propanediol. Cu-based catalysts require the selective cleavage of the secondary C–O bond against the cleavage of the C–C bond in the hydrogenolysis of glycerol in order to produce 1,2-propanediol. Acid-catalyzed glycerol dehydration and metal-catalyzed intermediate hydrogenation are the two steps in glycerol hydrogenolysis. Glycerol hydrogenolysis has been primarily attempted in the liquid phase over different metal catalysts synthesized via the impregnation, co-precipitation, solid combustion and decomposition of metal–organic frameworks.
1. Introduction
Glycerol or glycerin is a valuable byproduct in biodiesel production. Biodiesel can generate about 10 wt% glycerol as the main byproduct using hydrolysis and transesterification reactions. Crude waste effluents produced from biodiesel refineries contain glycerol, water, methanol and free fatty acids as well as organic and inorganic salts [38]. Glycerol and its derivatives have found diverse value-added applications in many industrial sectors such as pharmaceuticals, personal care, food processing, resins, explosives, cellophane, detergents, fabrics and others.
Pure glycerol is a sugary, colorless, odorless, viscous, non-toxic, biodegradable and hygroscopic liquid at room temperature. It is completely soluble in water due to the presence of three hydroxyl groups. Although it is chemically stable, glycerol is a reactive molecule owing to the formation of primary and secondary aromatic compounds. The glycerol backbone is central to all lipids known as triglycerides. The thermophysical properties of glycerol and polyethylene glycol have been investigated based on the deep eutectic solvent [40]. It was found that the use of deep eutectic solvent increased the thermal conductivity of glycerol with increasing temperature. In another study conducted by Hu et al. [41], the density of crude glycerol samples changed due to the presence of fatty acids methyl esters and water. Furthermore, the viscosity of five crude glycerol samples varied between 15 and 1213 mPa.s owing to their different compositions.
Glycerol has many applications, but one route of its use is in the synthesis of other chemicals. One of the possible chemicals that can be produced is 1,2-propanediol, commonly referred to as ethylene glycol. 1,2-propanediol has applications as a solvent, antifreeze agent and additive for food and cosmetic products [
54]. The market for 1,2-propanediol was globally valued at $4.35 billion in 2022 and is expected to grow at a compound annual growth rate of 6.1% until 2030 due to increasing demand [
55]. Like its counterpart, 1,3-propanediol can be synthesized from glycerol and has applications as a monomer to produce chemicals such as polyurethanes, polyethers and polytrimethylene terephthalate [
56]. Both 1,2-propanediol and 1,3-propanediol are used for many applications such as pharmaceuticals, artificial flavors, paints, urethane foams, cosmetics, fragrances and synthetic resins [
57].
The market for 1,3-propanediol was reported as being $433 million in 2021 and is projected to grow with a compound annual growth rate of 11.5% until 2030 [
58].
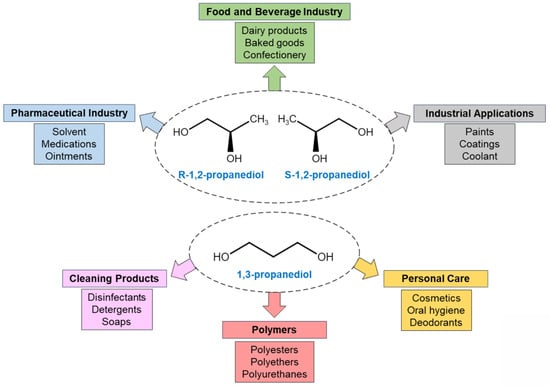
Figure 6 highlights significant industrial applications for 1,2-propanediol and 1,3-propanediol, such as in polymer production, as an additive in the food and medicinal drug industry, and as a solvent. The production of these two chemicals provides another value-added use for glycerol.
Figure 6. Chemical structure and applications of 1,2-propanediol and 1,3-propanediol.
Alternative sustainable routes for the production of 1,2-propanediol and 1,3-propanediol have been widely researched, especially through thermocatalytic and biocatalytic techniques, in order to aid the conversion of glycerol. A common catalytic method is hydrogenolysis [
59]. Different catalytic systems are also utilized, examples of which are Pt/W/β, the Pt/deAl-β core and Mg(OH)
2 shell catalyst and the Pt-In alloy catalyst [
60,
61,
62].
Several microorganisms have also been investigated in order to ferment glycerol and sugar substrates into 1,2-propanediol and 1,3-propanediol, examples of which include
Citrobacter freundii,
Clostridium beijerinckii,
Clostridium butyricum,
Clostridium diolis,
Clostridium pasteurianum,
Escherichia coli,
Klebsiella pneumoniae,
Lactobacillus panis and
Lactobacillus reuteri [
63,
64,
65,
66,
67,
68,
69,
70,
71].
Lactobacillus spp. involved in the fermentative production of propanediol are generally regarded as safe (GRAS) to handle compared to
Klebsiella spp. and
Escherichia spp., which are considered pathogenic unless specific containment measures are ensured.
2. Thermocatalytic Conversion of Glycerol to Propanediol
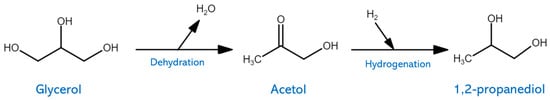
Significant research efforts have been focused on the catalytic transformation of glycerol for the synthesis of value-added chemicals owing to the rising prices of petroleum resources. Glycerol is an important byproduct due to its application to produce acrolein, glyceric acid, glycerol carbonate, and propanediol. As shown in
Figure 7, Cu-based catalysts require the selective cleavage of the secondary C–O bond against the cleavage of the C–C bond in the hydrogenolysis of glycerol in order to produce 1,2-propanediol [
82]. Acid-catalyzed glycerol dehydration and metal-catalyzed intermediate hydrogenation are the two steps in glycerol hydrogenolysis [
83]. Glycerol hydrogenolysis has been primarily attempted in the liquid phase over different metal catalysts synthesized via the impregnation, co-precipitation, solid combustion and decomposition of metal–organic frameworks. Some recent achievements in the selective hydrogenolysis of glycerol over bifunctional-supported catalysts for propanediol synthesis are summarized in
Table 3.
Figure 7. Schematic representation of hydrogenolysis of glycerol into 1,2-propanediol.
Table 3. Performance of various monometallic and bimetallic catalysts for hydrogenolysis of glycerol.
| Catalyst |
Preparation Method |
Process Conditions |
Main Observations |
Reference |
| 10Nb/Pd-Zr-Al |
Impregnation |
|
-
Glycerol conversion: 66.4%
-
1,2-propanediol selectivity: 81.3%
-
1,2-propanediol yield: 53.9%
|
Cai et al. [84] |
| 15Co0.5Cu/TiO2 |
Co-impregnation |
|
-
Glycerol conversion: 60.2%
-
1,2-propanediol selectivity: 86%
-
1,2-propanediol yield: 51.7%
|
Mondach et al. [85] |
| Copper carbide composite |
Co-precipitation |
|
-
Glycerol conversion: 79.3%
-
1,2-propanediol selectivity: 60.5%
-
1,2-propanediol yield: 47.9%
|
Liu et al. [83] |
| Cu/Al2O3 |
Wet impregnation |
|
-
Glycerol conversion: 28%
-
1,2-propanediol selectivity: 66.8%
-
1,2-propanediol yield: 18.7%
|
Azri et al. [86] |
| Cu/Mg-supported SiO2 |
Chemisorption-hydrolysis |
|
-
Glycerol conversion: 73.1%
-
1,2-propanediol selectivity: 88.2%
-
1,2-propanediol yield: 63.4%
|
Kumar et al. [87] |
| Cu-Al-Zn |
Co-precipitation |
|
-
Glycerol conversion: 43%
-
1,2-propanediol selectivity: 69%
-
1,2-propanediol yield: 29.6%
|
Mishra et al. [88] |
| CuNi30MgAl |
Wet impregnation |
|
-
Glycerol conversion: 65%
-
1,2-propanediol selectivity: 38.4%
-
1,2-propanediol yield: 25%
|
Mendonça et al. [89] |
| Ni/Dolomite |
Impregnation |
|
-
Glycerol conversion: 69.5%
-
1,2-propanediol selectivity: 52.7%
-
1,2-propanediol yield: 36.6%
|
Azri et al. [90] |
| Pt/WO3/zirconium phosphate |
Sequential wet impregnation |
|
-
Glycerol conversion: 68.4%
-
1,2-propanediol selectivity: 38.5%
-
1,2-propanediol yield: 26.2%
|
Bhowmik et al. [91] |
| Pt-In |
Successive impregnation method |
|
-
Glycerol conversion: 88%
-
1,2-propanediol selectivity: 82%
-
1,2-propanediol yield: 72%
|
Zhang et al. [62] |
| Ru-Cu/CaO-ZrO2 |
Successive incipient wetness impregnation |
|
-
Glycerol conversion: 8%
-
1,2-propanediol selectivity: 87%
-
1,2-propanediol yield: 69.6%
|
Salgado et al. [92] |
Bellè et al. [
59] found that carbon-supported bimetallic Ru-WO
x catalysts act effectively for the low-pressure hydrogenolysis of aqueous glycerol into propylene glycol. It was reported that WO
x clusters on the carbon surface resulted in chiral Brønsted acid sites, improving selectivity towards 1,2-propanediol (88%). Kunthakudee et al. [
93] observed the synergistic effects of CuAl
2O
4-CuO-Al
2O
3 nanocatalysts with various mole ratios of Cu/Al for glycerol hydrogenolysis. It was found that nanoparticle agglomeration was induced by Cu species during the flame process. Higher Cu content led to improved catalytic activity due to the high dispersion of Cu nanoparticles on a high surface area of Al
2O
3. On the other hand, alumina and Cu species acted as active sites and contributed to the hydrogenation of methyl lactate into 1,2-propanediol. Wei et al. [
94] evaluated a series of catalysts intercalated using Cu-Zn-Fe hydrotalcite-like compounds for glycerol hydrogenolysis. The catalytic performance for 1,3-propanediol production was greatly improved, with changes corresponding to the surface structure and physicochemical properties of the catalytic materials.
Sharma et al. [
95] performed catalytic hydrogenolysis of glycerol using mixed-oxide catalysts and reported a high propylene glycol yield. The used metal oxide catalysts were separated and regenerated up to four runs. However, hydrogenolysis of glycerol in the liquid phase required high operating pressures of 4–8 MPa. Kinetic parameters such as apparent activation energy can also help with the commercialization of hydrogenation reaction for the formation of acetol and propanediol [
96]. Recently, Khandelwal et al. [
35] demonstrated supercritical water gasification as a sustainable process for the conversion of crude glycerol into hydrogen-rich syngas at optimal temperature, pressure, reaction time and feed concentration conditions. Lastly, techno-economic assessment studies can determine the scalability of different thermocatalytic, biocatalytic or integrated processes for the commercial valorization of crude glycerol into biofuels and biochemicals [
97].
Other thermocatalytic conversion techniques such as single-atom catalysts, metal-organic frameworks and graphene matrices are gaining popularity for the valorization of waste feedstocks [
98,
99,
100,
101,
102,
103]. Recently, the selective transformation of biomass-derived feedstocks into value-added materials using single-atom catalysts has become one of the interesting topics in this field. The selective hydrogenolysis of glycerol into 1,2-propanediol using PtCu/MgAl-mixed metal oxides has been investigated [
100]. The authors suggested that 1,3-propanediol is selectively produced due to rising Brønsted acidity and the stabilization of secondary carbocation. Similarly, Xiong et al. [
103] reported the selective C–C cleavage of glycerol into value-added products using single-atom photocatalyst Ni/TiO
2. The nickel single-atom catalyst showed a high selectivity of 60% to glycolaldehyde under ambient conditions driven by light irradiation.
Metal–organic frameworks (MOFs), due to their adjustable topological structures and high surface area, can be used for catalytic oxidation of glycerol under nanoalkaline conditions. Ke et al. [
102] employed Pt-supported on an MOF-derived carbon nanosheet (Pt/Zr@NPCN) catalyst for the selective oxidation of glycerol into glyceric acid showing high catalytic activity and selectivity. It was suggested that a possible conversion of glycerol into glyceric acid and dihydroxyacetone can occur due to the side reaction of oxidation decarboxylation.
Due to its outstanding electrical conductivity, thermally expanded graphene oxide (TEGO) can be employed as a catalyst for the selective oxidation of glycerol. Yang et al. [
98] used Pt-Au alloy nanoparticles based on TEGO with various compositions for the aerobic oxidation of glycerol in a base-free aqueous solution. It was found that by increasing the Pt/Au ratio in TEGO-supported Pt-Au bimetallic catalysts, the selectivity of glyceric acid was reduced significantly due to the agglomeration of Pt particles.
Glycerol conversion into chemicals and the presence of fuel additives integrating routes within a biorefinery can lead to biodiesel production efficiency, sustainability and profitability using vegetable oils and waste fats. To compare novel glycerol conversion methods with conventional glycerol biorefinery techniques, energy and waste streams together with lifecycle metrics should be considered. Furthermore, efficient catalyst design by altering the Brønsted-to-Lewis acid site ratio of the oxide can change propanediol yield. The lifecycle and economic aspects of glycerol hydrogenation are determined based on the use of a catalyst for at least ten reaction cycles before its replacement [
104]. To maximize profitability and environmental benefits, the integration of glycerol conversion processes with other waste recovery processes should be considered. However, glycerol resources such as biodiesel production and the food industry supply chain are dependent on other processes. To consider an integrated biorefinery plant, glycerol needs to be an abundant and cheap waste raw material. The second step is minimizing the potential ecological risks associated with glycerol biorefinery systems to ensure sustainability [
105].
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9100894