Dysregulation of sulfur metabolism may result in elevated levels of volatile sulfur compounds (VSCs) in body fluids, breath, and/or excretions of cancer patients. Besides hydrogen sulfide (H2S), methanethiol is the predominant cancer-associated VSC and has been proposed as a promising biomarker for non-invasive cancer diagnosis. Gut bacteria are the major exogenous source of exposure to this foul-smelling toxic gas, with methanethiol-producing strains such as Fusobacterium nucleatum highly abundant in the gut microbiome of colorectal carcinoma (CRC) patients. Physiologically, methanethiol becomes rapidly degraded through the methanethiol oxidase (MTO) activity of selenium-binding protein 1 (SELENBP1). However, SELENBP1, which is considered a tumor suppressor, is often downregulated in tumor tissues, and this has been epidemiologically linked to poor clinical outcomes. In addition to impaired removal, an increase in methanethiol levels may derive from non-enzymatic reactions, such as a Maillard reaction between glucose and methionine, two metabolites enriched in cancer cells. High methionine concentrations in cancer cells may also result in enzymatic methanethiol production in mitochondria. Moreover, enzymatic endogenous methanethiol production may occur through methyltransferase-like protein 7B (METTL7B), which is present at elevated levels in some cancers, including CRC and hepatocellular carcinoma (HCC). In conclusion, methanethiol contributes to the "scent of cancer" as part of the cancer-associated signature combination of volatile organic compounds (VOCs).
- methyl mercaptan
- SELENBP1
- MTO
- microbiota
- H2S
- CRC
- volatile sulfur compounds
1. Introduction
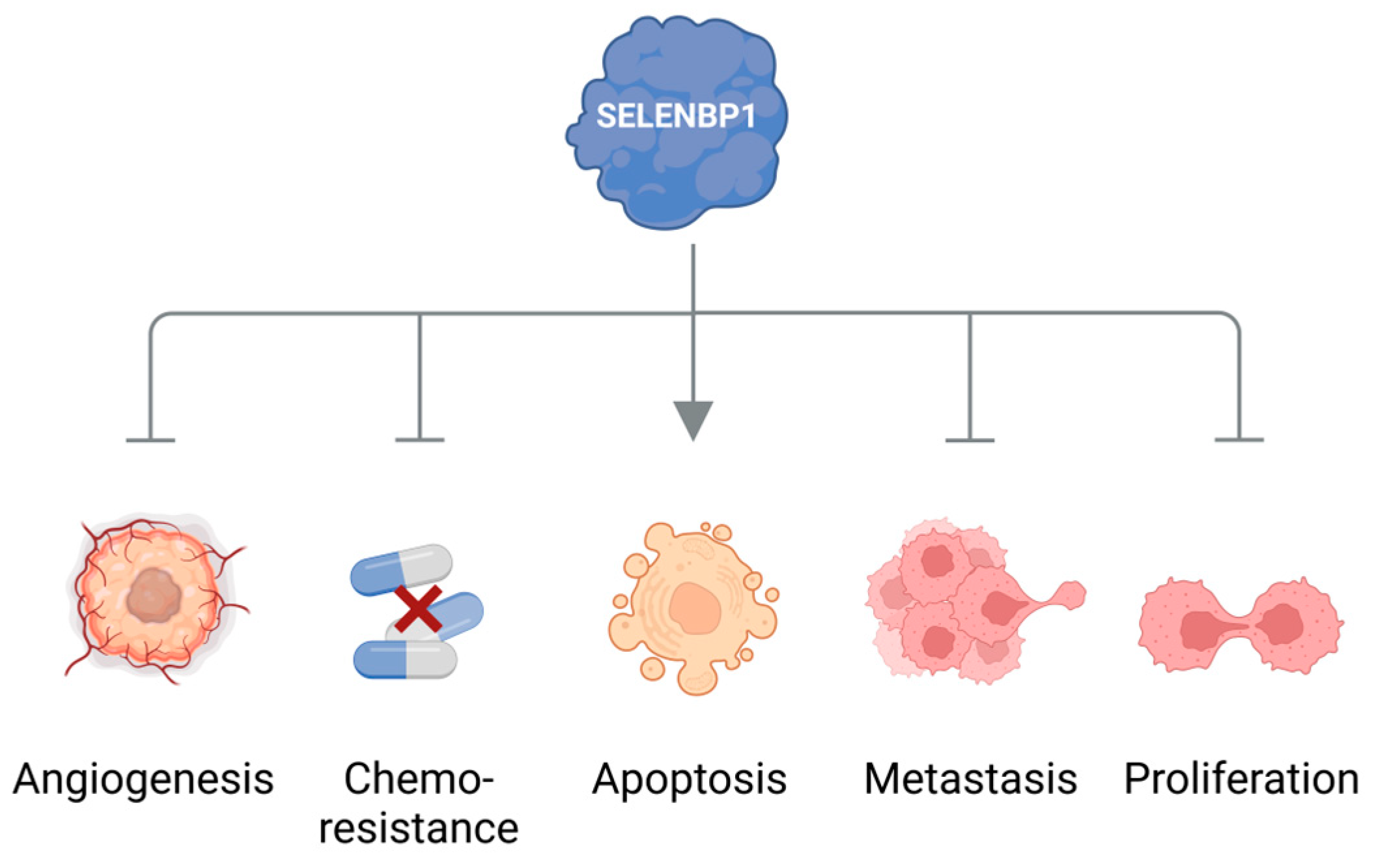
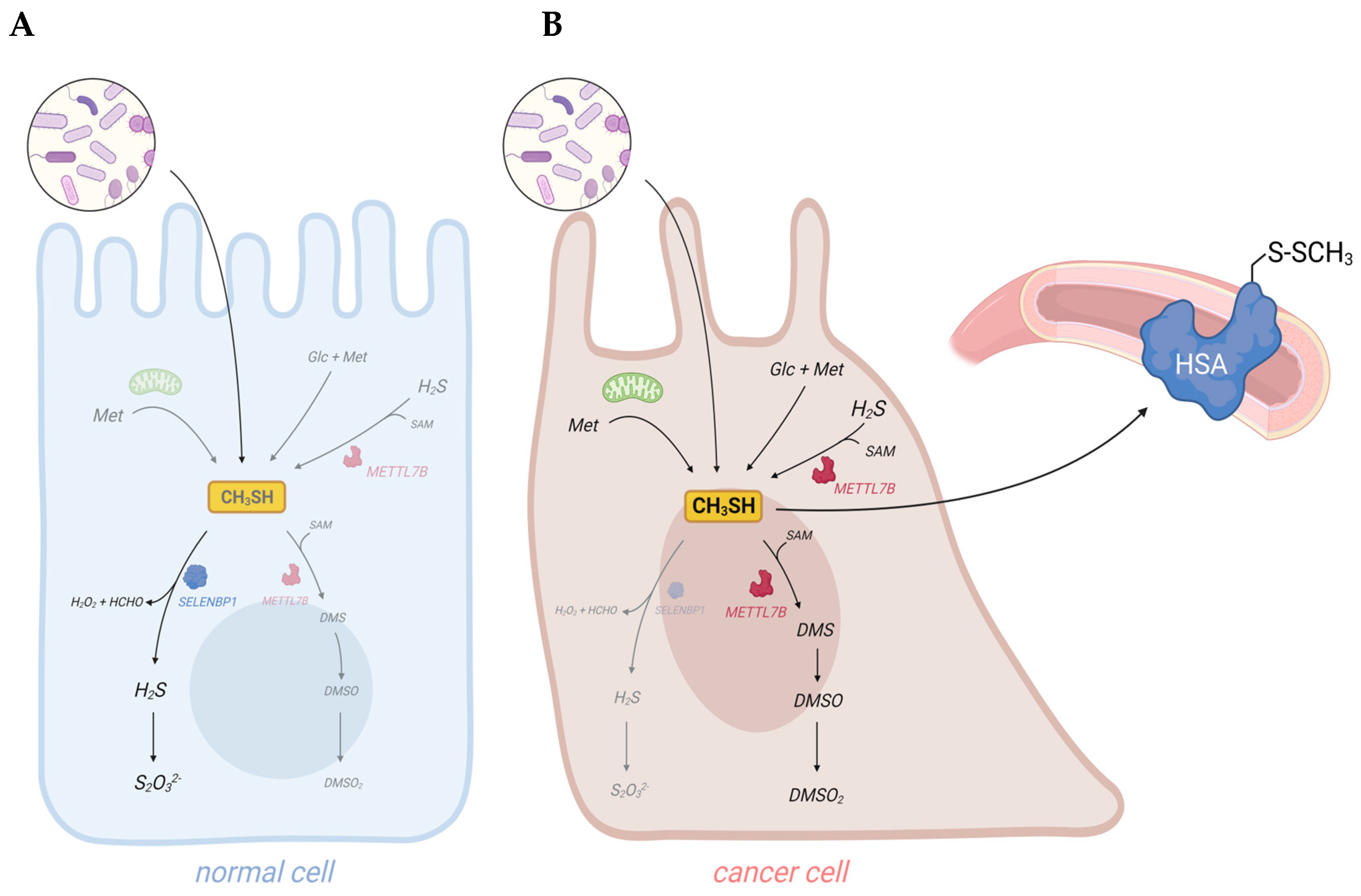
Cancer cells are discussed to be “addicted” to the sulfur-containing essentiell amino acid methionine [1]. The selective upregulation of the methionine transporter SLC43A2 allows cancer cells to outcompete cells in the tumor microenvironment for methionine supply [2]. Methionine is used for the biosynthesis of proteins, nucleotides, glutathione, and the methyl donor S-adenosylmethionine (SAM), which are required for cellular growth and proliferation, as well as protection against oxidative stress [1]. Moreover, methionine serves as a precursor for the endogenous and exogenous generation of two volatile sulfur compounds (VSCs): hydrogen sulfide (H2S) and methanethiol. Several strains of gut bacteria may convert dietary methionine to H2S and/or methanethiol, thus representing the major source of exposure of humans to both VSCs. In addition, H2S and methanethiol are produced endogenously through enzymatic and non-enzymatic synthesis [3][4]. The cancer-associated dysregulation of sulfur metabolism results in excess levels of both VSCs in tumor tissue as well as in body fluids, breath, and/or excretions of cancer patients that are increasingly being exploited for the establishment of convenient non-invasive screening methods to detect early signs of cancer [5][6][7][8]. In this regard, the dysregulated H2S biosynthesis in various types of cancer and the cancer-promoting effects of elevated H2S levels have recently been discussed in several reviews [4][9][10]. Excess H2S may promote cellular dedifferentiation and the growth of transformed cells as well as increase their potential to metastasize and develop resistance against chemotherapeutic agents [4]. The endogenous production of H2S in mammalian cells occurs largely through four enzymes: cystathionine β-synthase (CBS), cystathionine γ-lyase (CTH), 3-mercaptopyruvate sulfurtransferase (MPST), and selenium-binding protein 1 (SELENBP1) [3][11][12]. In particular, CBS has been shown to be upregulated in various types of cancer, including colorectal carcinoma (CRC), squamous cell carcinoma, and ovarian, breast, and thyroid cancers [4]. In several of those cancers, CTH and MPST levels were also found to be elevated [4]. On the other hand, SELENBP1, which generates H2S through the oxidation of methanethiol [12], is often downregulated in tumor tissue [13].
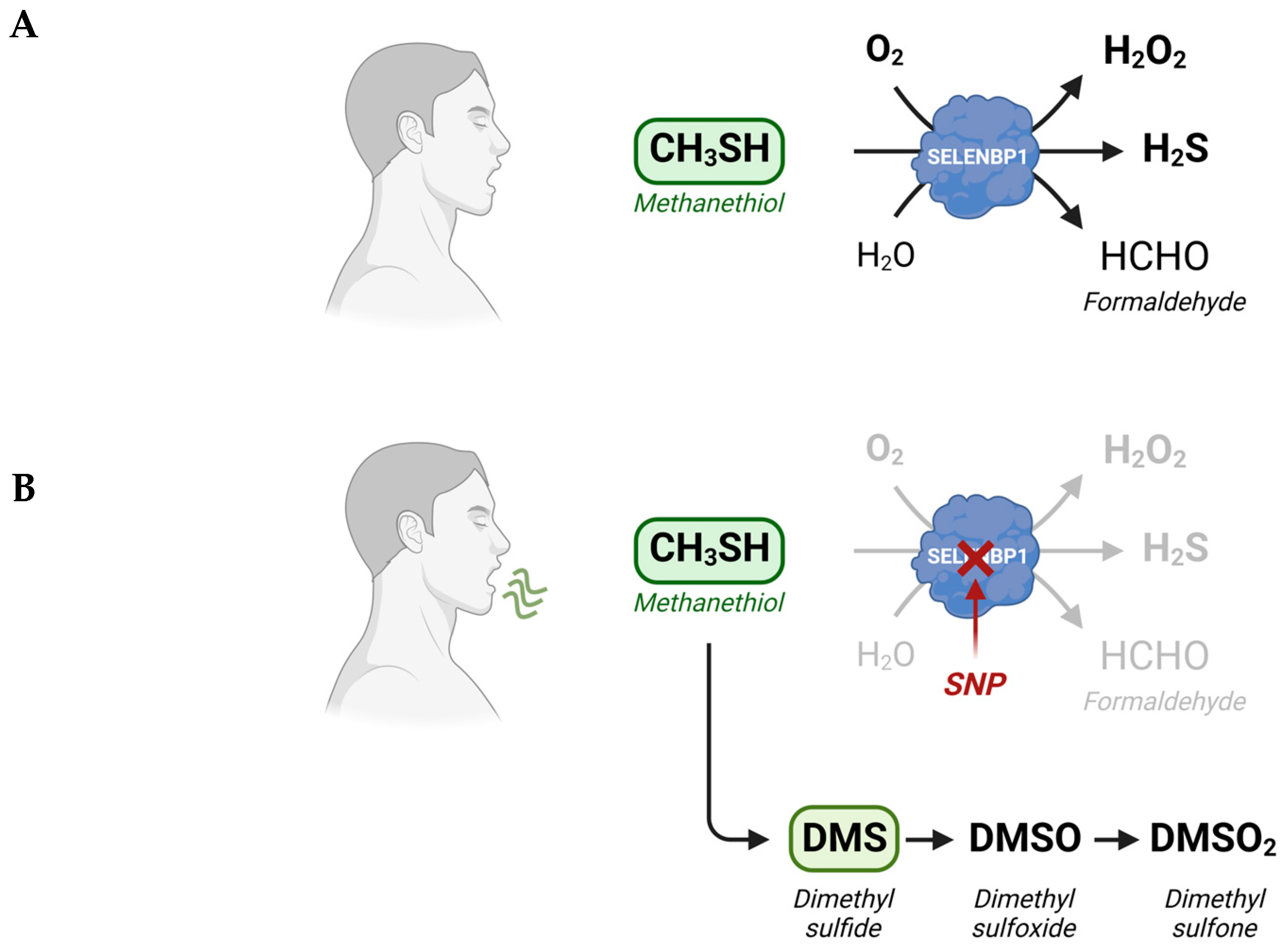
Methanethiol (methyl mercaptan, CH3SH) is an alkyl thiol and a member of a group of repulsive-smelling VSCs, characterized by their toxicity and low odor threshold. It is a colorless gas with a boiling point of 5.9 °C. The smell of methanethiol is reminiscent of rotten cabbage and is perceptible to humans at concentrations of ~1–2 parts per billion (ppb) [14]. A study on rats that investigated the toxicity of different VSCs reported a 24 h LD50 value of 675 parts per million (ppm) for acute exposure to methanethiol, which was similar to the LD50 of 444 ppm measured for H2S [15]. The toxicity of methanethiol has been attributed to its inhibiting effects on cytochrome c oxidase and the electron transfer in the respiratory chain [16]. Environmental microbial production of methanethiol occurs mainly through the methylation of H2S in the anoxic sediment/water interphase and through the elimination reactions of sulfur-containing amino acids catalyzed by L-methionine-γ-lyase (MGL). In addition, it can be generated from dimethylsulfoniopropionate (DMSP), an osmolyte in marine algae, through a coupled series of bacterial demethylation and cleavage reactions [14]. Although methanethiol has long been known as an environmental toxin and an intermediate in the biogeochemical sulfur cycle, its metabolism in humans has only recently gained more attention, following the identification of human selenium-binding protein 1 (SELENBP1) as a novel methanethiol-oxidizing enzyme in 2018 [12].
2. Exogenous and Endogenous Methanethiol Production in Humans
3. SELENBP1-Catalyzed Degradation of Methanethiol in Humans
4. Elevated Levels of Methanethiol and Its Derivatives in Various Types of Cancer
5. Conclusions and Outlook: Methanethiol as a Promising Biomarker for Non-Invasive Cancer Diagnosis
Taken together, elevated concentrations of methanethiol and its methylated derivatives in body fluids and excreted gases contribute to the “scent of cancer”, frequently observed in affected patients.
This entry is adapted from the peer-reviewed paper 10.3390/antiox12091780
References
- Sedillo, J.C.; Cryns, V.L. Targeting the methionine addiction of cancer. Am. J. Cancer Res. 2022, 12, 2249–2276.
- Bian, Y.; Li, W.; Kremer, D.M.; Sajjakulnukit, P.; Li, S.; Crespo, J.; Nwosu, Z.C.; Zhang, L.; Czerwonka, A.; Pawlowska, A.; et al. Cancer slc43a2 alters t cell methionine metabolism and histone methylation. Nature 2020, 585, 277–282.
- Scheller, A.S.; Philipp, T.M.; Klotz, L.O.; Steinbrenner, H. Altered capacity for h(2)s production during the spontaneous differentiation of caco-2 cells to colonocytes due to reciprocal regulation of cbs and selenbp1. Antioxidants 2022, 11, 1957.
- Shackelford, R.E.; Mohammad, I.Z.; Meram, A.T.; Kim, D.; Alotaibi, F.; Patel, S.; Ghali, G.E.; Kevil, C.G. Molecular functions of hydrogen sulfide in cancer. Pathophysiology 2021, 28, 437–456.
- Amal, H.; Ding, L.; Liu, B.B.; Tisch, U.; Xu, Z.Q.; Shi, D.Y.; Zhao, Y.; Chen, J.; Sun, R.X.; Liu, H.; et al. The scent fingerprint of hepatocarcinoma: In-vitro metastasis prediction with volatile organic compounds (vocs). Int. J. Nanomed. 2012, 7, 4135–4146.
- Bhatt, A.; Parsi, M.A.; Stevens, T.; Gabbard, S.; Kumaravel, A.; Jang, S.; Grove, D.; Lopez, R.; Murthy, S.; Vargo, J.J.; et al. Volatile organic compounds in plasma for the diagnosis of esophageal adenocarcinoma: A pilot study. Gastrointest. Endosc. 2016, 84, 597–603.
- Ishibe, A.; Ota, M.; Takeshita, A.; Tsuboi, H.; Kizuka, S.; Oka, H.; Suwa, Y.; Suzuki, S.; Nakagawa, K.; Suwa, H.; et al. Detection of gas components as a novel diagnostic method for colorectal cancer. Ann. Gastroenterol. Surg. 2018, 2, 147–153.
- Xie, X.; Yu, W.; Chen, Z.; Wang, L.; Yang, J.; Liu, S.; Li, L.; Li, Y.; Huang, Y. Early-stage oral cancer diagnosis by artificial intelligence-based sers using ag nws@zif core-shell nanochains. Nanoscale 2023, 15, 13466–13472.
- Ascencao, K.; Szabo, C. Emerging roles of cystathionine beta-synthase in various forms of cancer. Redox Biol. 2022, 53, 102331.
- Lin, H.; Yu, Y.; Zhu, L.; Lai, N.; Zhang, L.; Guo, Y.; Lin, X.; Yang, D.; Ren, N.; Zhu, Z.; et al. Implications of hydrogen sulfide in colorectal cancer: Mechanistic insights and diagnostic and therapeutic strategies. Redox Biol. 2023, 59, 102601.
- Kabil, O.; Banerjee, R. Enzymology of h2s biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782.
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; de Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; van den Heuvel, L.; Omran, H.; et al. Mutations in selenbp1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018, 50, 120–129.
- Elhodaky, M.; Diamond, A.M. Selenium-binding protein 1 in human health and disease. Int. J. Mol. Sci. 2018, 19, 3437.
- Schafer, H.; Eyice, O. Microbial cycling of methanethiol. Curr. Issues Mol. Biol. 2019, 33, 173–182.
- Tansy, M.F.; Kendall, F.M.; Fantasia, J.; Landin, W.E.; Oberly, R.; Sherman, W. Acute and subchronic toxicity studies of rats exposed to vapors of methyl mercaptan and other reduced-sulfur compounds. J. Toxicol. Environ. Health 1981, 8, 71–88.
- Vahlkamp, T.; Meijer, A.J.; Wilms, J.; Chamuleau, R.A. Inhibition of mitochondrial electron transfer in rats by ethanethiol and methanethiol. Clin. Sci. 1979, 56, 147–156.
- Suarez, F.L.; Springfield, J.; Levitt, M.D. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut 1998, 43, 100–104.
- He, X.; Slupsky, C.M. Metabolic fingerprint of dimethyl sulfone (dmso2) in microbial-mammalian co-metabolism. J. Proteome Res. 2014, 13, 5281–5292.
- Sato, D.; Nozaki, T. Methionine gamma-lyase: The unique reaction mechanism, physiological roles, and therapeutic applications against infectious diseases and cancers. IUBMB Life 2009, 61, 1019–1028.
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; de Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; van den Heuvel, L.; Omran, H.; et al. Mutations in selenbp1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018, 50, 120–129.
- Scheller, A.S.; Philipp, T.M.; Klotz, L.O.; Steinbrenner, H. Altered capacity for h(2)s production during the spontaneous differentiation of caco-2 cells to colonocytes due to reciprocal regulation of cbs and selenbp1. Antioxidants 2022, 11, 1957.
- Maldonato, B.J.; Russell, D.A.; Totah, R.A. Human mettl7b is an alkyl thiol methyltransferase that metabolizes hydrogen sulfide and captopril. Sci. Rep. 2021, 11, 4857.
- Scislowski, P.W.; Pickard, K. The regulation of transaminative flux of methionine in rat liver mitochondria. Arch. Biochem. Biophys. 1994, 314, 412–416.
- Finkelstein, A.; Benevenga, N.J. The effect of methanethiol and methionine toxicity on the activities of cytochrome c oxidase and enzymes involved in protection from peroxidative damage. J. Nutr. 1986, 116, 204–215.
- Furne, J.; Springfield, J.; Koenig, T.; DeMaster, E.; Levitt, M.D. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: A specialized function of the colonic mucosa. Biochem. Pharmacol. 2001, 62, 255–259.
- Levitt, M.D.; Furne, J.; Springfield, J.; Suarez, F.; DeMaster, E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Investig. 1999, 104, 1107–1114.
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen sulfide oxidation by sulfide quinone oxidoreductase. Chembiochem 2021, 22, 949–960.
- Li, T.; Yang, W.; Li, M.; Byun, D.S.; Tong, C.; Nasser, S.; Zhuang, M.; Arango, D.; Mariadason, J.M.; Augenlicht, L.H. Expression of selenium-binding protein 1 characterizes intestinal cell maturation and predicts survival for patients with colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1289–1299.
- Philipp, T.M.; Gernoth, L.; Will, A.; Schwarz, M.; Ohse, V.A.; Kipp, A.P.; Steinbrenner, H.; Klotz, L.O. Selenium-binding protein 1 (selenbp1) is a copper-dependent thiol oxidase. Redox Biol. 2023, 65, 102807.
- Philipp, T.M.; Will, A.; Richter, H.; Winterhalter, P.R.; Pohnert, G.; Steinbrenner, H.; Klotz, L.O. A coupled enzyme assay for detection of selenium-binding protein 1 (selenbp1) methanethiol oxidase (mto) activity in mature enterocytes. Redox Biol. 2021, 43, 101972.
- Funato, K.; Abe, T.; Kurita, R.; Watanabe, Y.; Nakamura, Y.; Miyata, S.; Furukawa, Y.; Satake, M. Identification of characteristic proteins at late-stage erythroid differentiation in vitro. Hum. Cell 2021, 34, 745–749.
- Steinbrenner, H.; Micoogullari, M.; Hoang, N.A.; Bergheim, I.; Klotz, L.O.; Sies, H. Selenium-binding protein 1 (selenbp1) is a marker of mature adipocytes. Redox Biol. 2019, 20, 489–495.
- Ringrose, J.H.; van Solinge, W.W.; Mohammed, S.; O’Flaherty, M.C.; van Wijk, R.; Heck, A.J.; Slijper, M. Highly efficient depletion strategy for the two most abundant erythrocyte soluble proteins improves proteome coverage dramatically. J. Proteome Res. 2008, 7, 3060–3063.
- Randi, E.B.; Casili, G.; Jacquemai, S.; Szabo, C. Selenium-binding protein 1 (selenbp1) supports hydrogen sulfide biosynthesis and adipogenesis. Antioxidants 2021, 10, 361.
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of h(2)s signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337.
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748.
- Goubern, M.; Andriamihaja, M.; Nubel, T.; Blachier, F.; Bouillaud, F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007, 21, 1699–1706.
- McClain, C.J.; Zieve, L.; Doizaki, W.M.; Gilberstadt, S.; Onstad, G.R. Blood methanethiol in alcoholic liver disease with and without hepatic encephalopathy. Gut 1980, 21, 318–323.
- Stephen, A.S.; Dhadwal, N.; Nagala, V.; Gonzales-Marin, C.; Gillam, D.G.; Bradshaw, D.J.; Burnett, G.R.; Allaker, R.P. Interdental and subgingival microbiota may affect the tongue microbial ecology and oral malodour in health, gingivitis and periodontitis. J. Periodontal Res. 2021, 56, 1174–1184.
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20.
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306.
- Ishibe, A.; Ota, M.; Takeshita, A.; Tsuboi, H.; Kizuka, S.; Oka, H.; Suwa, Y.; Suzuki, S.; Nakagawa, K.; Suwa, H.; et al. Detection of gas components as a novel diagnostic method for colorectal cancer. Ann. Gastroenterol. Surg. 2018, 2, 147–153.
- Yamagishi, K.; Onuma, K.; Chiba, Y.; Yagi, S.; Aoki, S.; Sato, T.; Sugawara, Y.; Hosoya, N.; Saeki, Y.; Takahashi, M.; et al. Generation of gaseous sulfur-containing compounds in tumour tissue and suppression of gas diffusion as an antitumour treatment. Gut 2012, 61, 554–561.
- Kwon, I.J.; Jung, T.Y.; Son, Y.; Kim, B.; Kim, S.M.; Lee, J.H. Detection of volatile sulfur compounds (vscs) in exhaled breath as a potential diagnostic method for oral squamous cell carcinoma. BMC Oral Health 2022, 22, 268.
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200.
- Martinez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680.
- Sedillo, J.C.; Cryns, V.L. Targeting the methionine addiction of cancer. Am. J. Cancer Res. 2022, 12, 2249–2276.
- Guo, J.; Yang, Y.; Buettner, R.; Rosen, S.T. Targeting the methionine-methionine adenosyl transferase 2a- s -adenosyl methionine axis for cancer therapy. Curr. Opin. Oncol. 2022, 34, 546–551.
- Ye, D.; Jiang, Y.; Sun, Y.; Li, Y.; Cai, Y.; Wang, Q.; Wang, O.; Chen, E.; Zhang, X. Mettl7b promotes migration and invasion in thyroid cancer through epithelial-mesenchymal transition. J. Mol. Endocrinol. 2019, 63, 51–61.
- Elhodaky, M.; Diamond, A.M. Selenium-binding protein 1 in human health and disease. Int. J. Mol. Sci. 2018, 19, 3437.
- Caswell, D.R.; Chuang, C.H.; Ma, R.K.; Winters, I.P.; Snyder, E.L.; Winslow, M.M. Tumor suppressor activity of selenbp1, a direct nkx2-1 target, in lung adenocarcinoma. Mol. Cancer Res. 2018, 16, 1737–1749.
- Ying, Q.; Ansong, E.; Diamond, A.M.; Lu, Z.; Yang, W.; Bie, X. Quantitative proteomic analysis reveals that anti-cancer effects of selenium-binding protein 1 in vivo are associated with metabolic pathways. PLoS ONE 2015, 10, e0126285.
- Zeng, H.; Zhao, X.; Tang, C. Downregulation of selenbp1 enhances oral squamous cell carcinoma chemoresistance through keap1-nrf2 signaling. Cancer Chemother. Pharmacol. 2021, 88, 223–233.
- Zhang, X.; Hong, R.; Bei, L.; Yang, J.; Zhao, X.; Hu, Z.; Chen, L.; Meng, H.; Zhang, Q.; Niu, G.; et al. Selenium binding protein 1 inhibits tumor angiogenesis in colorectal cancers by blocking the delta-like ligand 4/notch1 signaling pathway. Transl. Oncol. 2022, 18, 101365.
- Zhu, Y.; Pu, Q.; Zhang, Q.; Liu, Y.; Ma, Y.; Yuan, Y.; Liu, L.; Zhu, W. Selenium-binding protein 1 inhibits malignant progression and induces apoptosis via distinct mechanisms in non-small-cell lung cancer. Cancer Med. 2023, 12, 17149–17170.
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581.
- Miller-Atkins, G.; Acevedo-Moreno, L.A.; Grove, D.; Dweik, R.A.; Tonelli, A.R.; Brown, J.M.; Allende, D.S.; Aucejo, F.; Rotroff, D.M. Breath metabolomics provides an accurate and noninvasive approach for screening cirrhosis, primary, and secondary liver tumors. Hepatol. Commun. 2020, 4, 1041–1055.
- Li, Z.; Shu, J.; Zhang, P.; Sun, W.; Yang, B.; Zhang, H. Real-time ultrasensitive vuv-pims detection of representative endogenous volatile markers in cancers. Cancer Biomark. 2016, 16, 477–487.
- Kwak, J.; Gallagher, M.; Ozdener, M.H.; Wysocki, C.J.; Goldsmith, B.R.; Isamah, A.; Faranda, A.; Fakharzadeh, S.S.; Herlyn, M.; Johnson, A.T.; et al. Volatile biomarkers from human melanoma cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 931, 90–96.
- Kybert, N.; Prokop-Prigge, K.; Otto, C.M.; Ramirez, L.; Joffe, E.; Tanyi, J.; Piltz-Seymour, J.; Johnson, A.T.C.; Preti, G. Exploring ovarian cancer screening using a combined sensor approach: A pilot study. AIP Adv. 2020, 10, 035213.
- Skorupa, A.; Ponski, M.; Ciszek, M.; Cichon, B.; Klimek, M.; Witek, A.; Pakulo, S.; Boguszewicz, L.; Sokol, M. Grading of endometrial cancer using (1)h hr-mas nmr-based metabolomics. Sci. Rep. 2021, 11, 18160.
- Sever, A.; Abd Elkadir, A.; Matana, Y.; Gopas, J.; Zeiri, Y. Biomarkers for detection and monitoring of b16 melanoma in mouse urine and feces. J. Biomark. 2015, 2015, 841245.
- Grigoryan, H.; Schiffman, C.; Gunter, M.J.; Naccarati, A.; Polidoro, S.; Dagnino, S.; Dudoit, S.; Vineis, P.; Rappaport, S.M. Cys34 adductomics links colorectal cancer with the gut microbiota and redox biology. Cancer Res. 2019, 79, 6024–6031.
- Shirasu, M.; Nagai, S.; Hayashi, R.; Ochiai, A.; Touhara, K. Dimethyl trisulfide as a characteristic odor associated with fungating cancer wounds. Biosci. Biotechnol. Biochem. 2009, 73, 2117–2120.
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266.
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449.
- Janssens, E.; van Meerbeeck, J.P.; Lamote, K. Volatile organic compounds in human matrices as lung cancer biomarkers: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 153, 103037.
- Wang, L.; Li, J.; Xiong, X.; Hao, T.; Zhang, C.; Gao, Z.; Zhong, L.; Zhao, Y. Volatile organic compounds as a potential screening tool for neoplasm of the digestive system: A meta-analysis. Sci. Rep. 2021, 11, 23716.
