Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The gut microbiota (GM) functions as an endocrine organ that can influence other distant organs. The GM has been found to modulate hormone levels in the body, especially estrogens in women
- gut microbiota
- gynecological disorders
1. Introduction
Humans are ‘superorganisms’ that are colonized by 1014 total cells of more than 1000 species of bacteria, especially in the gastrointestinal tract [1][2]. Each host has a unique microbiota, which is essential for its immunity, nutrition, and pathogenesis [3][4]. Microorganisms inhabit various anatomical sites in the body such as the skin, mucosa, gastrointestinal tract, respiratory tract, urogenital tract, and mammary gland. They establish complex and distinct ecosystems that adapt to the specific environmental circumstances of each niche [5].
Starting from birth, a strict symbiotic relationship between the human body and its native microbiota is established. This relationship plays crucial functions in maintaining overall health and well-being. Therefore, its pivotal roles in protecting against pathogens; regulating metabolic, endocrine, and immune processes; and in influencing drug metabolism and absorption have started to be elucidated [5]. The microbiota leads the body to experience various changes from conception to death and also undergoes continuous modifications throughout life, which are influenced by various host elements such as age, dietary habits, lifestyle, hormonal fluctuations, and medical conditions [6]. Nevertheless, alterations in the microbiota composition, known as dysbiosis, have the potential to result in severe and even life-threatening illnesses, including gynecological disorders [7][8]. In detail, the gut microbiota (GM) functions as an endocrine organ that can influence other distant organs such as the central nervous system and the liver. It has been demonstrated that many chronic conditions, such as obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD), are all linked to GM dysbiosis [9]. Recent investigations suggest that intestinal dysbiosis can affect gut permeability, the innate immune system, the fermentation of indigestible carbohydrates, and the intestinal production of short-chain fatty acids (SCFAs), which can lead to NAFLD [10][11]. Consequently, NAFLD is related to other diseases like diabetes mellitus, obesity, metabolic syndrome, hypertension, renal disorders, and cardiovascular diseases—pathologies that strongly affect human life [12][13][14].
SCFAs are a group of fatty acids produced in the colon by the bacterial fermentation of dietary fibers and resistant starch. The most common types are butyrate, propionate, and acetate. SCFAs allow the growth of homeostasis-promoting bacteria, such as Lactobacilli and Bifidobacteria, and hinders colonization by opportunistic pathogenic bacteria, including Clostridium and Escherichia coli [15]. In addition, SCFAs contribute to the preservation of a functional gut barrier and to the maintenance of host homeostasis by stimulating the regeneration of epithelial cells and the production of mucus and antimicrobial peptides [16]. Other critical SCFAs roles are the modulation of T regulatory (Treg) cells, as well as their crucial physiological effects on several organs, including the brain and the urogenital apparatus [17][18][19]. G-protein coupled receptors (GPCR) and free fatty acid receptors (FFA) are the main receptor types that are activated by SCFAs; they are expressed in several tissues and regulate both energy metabolism and immune response [20]. Growing evidence suggests that intestinal dysbiosis can cause various immune and metabolic alterations through the activity of bacterial products: a decrease in SCFAs’ production can increase the risk of intestinal, neurological, and cardiovascular diseases development [21][22][23][24]. Dysbiosis can also increase lipopolysaccharide (LPS) circulating levels that can enhance the inflammatory response through micro-organism-associated molecular pattern (MAMPs) and the activation of pattern recognition receptors (PRR) such as toll-like receptors (TLRs) signaling pathways [25][26] (Figure 1). Moreover, the production of pro-inflammatory cytokines, including interleukin-17 (IL-17), tumor necrosis factor-α (TNF-α), and interferon-γ (INF-γ), can result in reactive oxygen species (ROS) release in the cells of distant organs, including the gynecological tract, whose homeostasis could be altered, contributing to the induction of a pathological state [27] (Figure 1). In addition to SCFAs, there are several metabolites produced by the microbiota that are involved in the gut-brain axis modulation, such as neurotransmitters [28] (Figure 1).
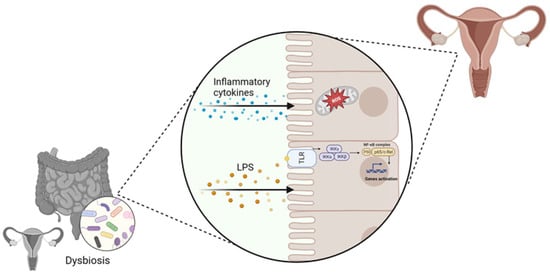
Figure 1. Involvement of vaginal and gut dysbiosis in the alteration of distal tissues homeostasis, contributing to gynecological disorders’ development (created with Biorender.com, accessed on 22 September 2023).
2. Gut Microbiome–Estrogen Axis in Gynecological Disorders
It has been widely recognized that the mutual relationships and metabolic activities among microorganisms have a significant impact on the host pathophysiology [28]. In particular, the gut microbiome (GM) has been found to modulate hormone levels in the body, especially estrogens in women [29]. The connection between GM and estrogen was first observed thirty years ago when Adlercreutz et al. found that antibiotics assumption reduced estrogen levels in women [30]. The GM mainly controls estrogen levels through the secretion of an enzyme called β-glucuronidase, which is encoded by several GM genera, including Bacteroides, Bifidobacterium, Escherichia, and Lactobacillus [31]. This enzyme converts conjugated estrogens to deconjugated forms in the gastrointestinal tract. These deconjugated and unbound “active” estrogens enter the bloodstream and subsequently act on estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), eliciting downstream activation of intracellular signaling cascades, gene transcription, and epigenetic effects [29][32]. When there is a decrease in β-glucuronidase activity due to an imbalance in the GM community (dysbiosis), there is less estrogen deconjugation, resulting in lower circulating estrogen levels [33]. Conversely, increased β-glucuronidase activity can increase estrogen levels. Thus, maintaining optimal β-glucuronidase activity is critical for regulating estrogen levels in females [34].
Estrogens contribute to epithelial proliferation throughout the female reproductive system and have been shown to drive proliferative diseases such as endometriosis and polycystic ovary syndrome (PCOS) [8][35].
Endometriosis is defined as the presence of endometrial tissue outside the uterus, including glands and stroma, that express the ER and therefore respond to estrogen. Endometriosis is a benign chronic inflammatory gynecological disease, but it can also involve the malignant behavior of invasion and migration [36]. Some studies have found higher levels of taxa that encode for β-glucuronidase, which are mainly Bifidobacterium and Escherichia, in endometriosis women compared with control women [37][38]. Wei Y. et al. found that β-glucuronidase promoted endometriosis development directly or indirectly by causing macrophage dysfunction. They documented the GM changes on patients and mice with endometriosis and the effect of β-glucuronidase on the proliferation and invasion of endometrial stromal cells and the development of endometriotic lesions [39].
The correlation between GM and hormones regulation was also found to affect PCOS disease [40]. PCOS is a common endocrine disorder in women of reproductive age and its clinical features are mainly oligo-ovulation or anovulation, hyperandrogenemia and insulin resistance. Moreover, PCOS is considered one of the leading causes of infertility in women of childbearing age [41][42]. The etiology and pathogenesis of PCOS remain unclear and may be multi-factorial, but in recent years growing evidence has highlighted a GM role in modulating PCOS progression.
In 2012, Tremellen et al. hypothesized that the gut flora could be related to PCOS, suggesting that GM imbalance could be associated with various PCOS clinical symptoms, such as hyperandrogenemia, multiple ovarian cysts, and anovulation [43]. Since then, several studies have found that specific microflora was changed in PCOS patients compared to healthy controls, such as the variation in the balance between Bacteroides and Firmicutes [40][44][45]. Moreover, the increase in the relative abundance of Firmicutes and Bacteroidetes was positively correlated with androgens concentration, body mass index, and insulin resistance, as well as with the level of free testosterone [46][47]. Regarding the genera Bacteroides, Liu et al. observed an increase in Escherichia and Shigella in PCOS patients, and a similar GM composition compared to obese control women [48]. The GM modifications in PCOS are different, sometimes controversial, and not yet fully understood.
Anyway the GM may influence the sex hormones levels also by the production of SCFAs [49]. These SCFAs have been shown to exert anti-proliferative effects [50][51] and have some anti-inflammatory properties that can be extended to distant organs [52]. SCFAs primarily affect the cells through several mechanisms, including the activation of G-protein-coupled receptors, namely, GPR43, GPR41, and GPR109A [53], which are known to downregulate inflammation [54]; and the inhibition of histone deacetylases [55], and the inhibition of histone deacetylases [56]
In an interesting in vitro study, porcine granulosa cells treated with low concentrations of butyric acid showed increased progesterone secretion, while higher butyrate concentrations significantly inhibited the progesterone secretion via the cAMP signaling pathway [48]. Another study by Liu et al. showed that the gut-derived butyrate can contribute to nonalcoholic fatty liver disease in premenopausal women with estrogen deficiency [56]. Moreover, it has been observed that fecal samples of mice affected by endometriosis contained lower levels of SCFAs and butyrate compared with control mice, and treatment with butyrate resulted in a decrease in the growth of both mouse and human endometriotic lesions [57][58].
There is evidence of lower concentrations of SCFAs in fecal samples from PCOS patients [59]. Indeed, probiotics’ supplementation promoted the growth of Faecalibacterium prausnitzii, Bifidobacterium, and Akkermansia, which are SCFA-producing bacteria, and can lead to an increase in intestinal SCFAs. In turns, SCFAs bind to their receptors on enteroendocrine cells and directly stimulate the release of gut–brain mediators that can influence sex hormone secretion by the pituitary gland and hypothalamus via the gut–brain axis [60] (Figure 2).
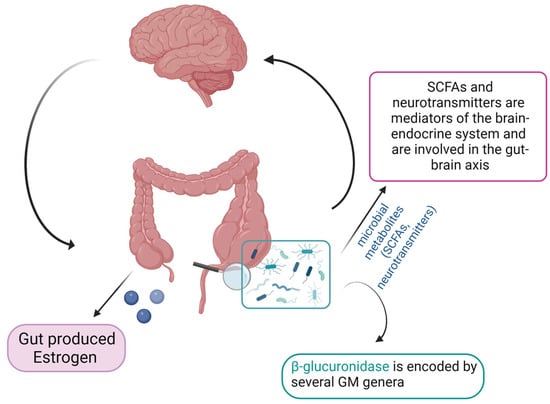
Figure 2. Schematic representation of the gut microbiome–estrogen axis (created with Biorender.com, accessed on 22 September 2023).
These studies confirm that the GM metabolites produced in response to dietary intake may play a role in regulating estrogen and progesterone levels in women. In light of these data, GM relevance in female gynecological disorders and in conditions affecting the reproductive tract must be taken into consideration [61][62].
While investigations in mice are starting to propose ways in which the GM impacts female gynecological disorders, no causal relationships between the GM and such disorders have been established in humans [33]. The significance of specific GM metabolites derived from the gut in relation to female reproductive health remains unknown. However, this field of study holds promise as it could lead to dietary interventions and/or fecal microbiota transplantation to improve the impact of gynecological disorders.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11102407
References
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118.
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438.
- Johnson, K.V. Gut microbiome composition and diversity are related to human personality traits. Hum. Microb. J. 2020, 15, 100069.
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705.
- Ding, T.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360.
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812.
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302.
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53.
- Naghipour, A.; Amini-Salehi, E.; Orang Gorabzarmakhi, M.; Shahdkar, M.; Fouladi, B.; Alipourfard, I.; Sanat, Z.M. Effects of gut microbial therapy on lipid profile in individuals with non-alcoholic fatty liver disease: An umbrella meta-analysis study. Syst. Rev. 2023, 12, 144.
- Moschen, A.R.; Kaser, S.; Tilg, H. Non-alcoholic steatohepatitis: A microbiota-driven disease. Trends Endocrinol. Metab. 2013, 24, 537–545.
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411.
- Cao, Y.; Deng, Y.; Wang, J.; Zhao, H.; Zhang, J.; Xie, W. The association between NAFLD and risk of chronic kidney disease: A cross-sectional study. Ther. Adv. Chronic Dis. 2021, 12, 20406223211048649.
- Ma, C.; Yan, K.; Wang, Z.; Zhang, Q.; Gao, L.; Xu, T.; Sai, J.; Cheng, F.; Du, Y. The association between hypertension and nonalcoholic fatty liver disease (NAFLD): Literature evidence and systems biology analysis. Bioengineered 2021, 12, 2187–2202.
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612.
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184.
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979.
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155.
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573.
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210.
- Baldi, S.; Menicatti, M.; Nannini, G.; Niccolai, E.; Russo, E.; Ricci, F.; Pallecchi, M.; Romano, F.; Pedone, M.; Poli, G.; et al. Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease. Nutrients 2021, 13, 742.
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558.
- De Marchi, F.; Munitic, I.; Amedei, A.; Berry, J.D.; Feldman, E.L.; Aronica, E.; Nardo, G.; Van Weehaeghe, D.; Niccolai, E.; Prtenjaca, N.; et al. Interplay between immunity and amyotrophic lateral sclerosis: Clinical impact. Neurosci. Biobehav. Rev. 2021, 127, 958–978.
- Niccolai, E.; Di Pilato, V.; Nannini, G.; Baldi, S.; Russo, E.; Zucchi, E.; Martinelli, I.; Menicatti, M.; Bartolucci, G.; Mandrioli, J.; et al. The Gut Microbiota-Immunity Axis in ALS: A Role in Deciphering Disease Heterogeneity? Biomedicines 2021, 9, 753.
- Lim, R.; Barker, G.; Lappas, M. The TLR2 ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-κB-dependent signalling. Am. J. Reprod. Immunol. 2014, 71, 401–417.
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977.
- Mirmonsef, P.; Zariffard, M.R.; Gilbert, D.; Makinde, H.; Landay, A.L.; Spear, G.T. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am. J. Reprod. Immunol. 2012, 67, 391–400.
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59.
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253.
- Adlercreutz, H.; Pulkkinen, M.O.; Hämäläinen, E.K.; Korpela, J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid Biochem. 1984, 20, 217–229.
- Candeliere, F.; Raimondi, S.; Ranieri, R.; Musmeci, E.; Zambon, A.; Amaretti, A.; Rossi, M. β-Glucuronidase Pattern Predicted from Gut Metagenomes Indicates Potentially Diversified Pharmacomicrobiomics. Front. Microbiol. 2022, 13, 826994.
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552.
- Chadchan, S.B.; Singh, V.; Kommagani, R. Female reproductive dysfunctions and the gut microbiota. J. Mol. Endocrinol. 2022, 69, R81–R94.
- Siddiqui, R.; Makhlouf, Z.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Gut Microbiome and Female Health. Biology 2022, 11, 1683.
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599.
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256.
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204.
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616.
- Wei, Y.; Tan, H.; Yang, R.; Yang, F.; Liu, D.; Huang, B.; OuYang, L.; Lei, S.; Wang, Z.; Jiang, S.; et al. Gut dysbiosis-derived β-glucuronidase promotes the development of endometriosis. Fertil. Steril. 2023, 120, 682–694.
- Liu, J.; Liu, Y.; Li, X. Effects of intestinal flora on polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1151723.
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284.
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15.
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112.
- Wang, T.; Sha, L.; Li, Y.; Zhu, L.; Wang, Z.; Li, K.; Lu, H.; Bao, T.; Guo, L.; Zhang, X.; et al. Dietary α-Linolenic Acid-Rich Flaxseed Oil Exerts Beneficial Effects on Polycystic Ovary Syndrome through Sex Steroid Hormones-Microbiota-Inflammation Axis in Rats. Front. Endocrinol. 2020, 11, 284.
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65.
- Zeng, X.; Xie, Y.J.; Liu, Y.T.; Long, S.L.; Mo, Z.C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221.
- Zeng, B.; Lai, Z.; Sun, L.; Zhang, Z.; Yang, J.; Li, Z.; Lin, J. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): A pilot study. Res. Microbiol. 2019, 170, 43–52.
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324.
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021, 12, 711137.
- Semaan, J.; El-Hakim, S.; Ibrahim, J.N.; Safi, R.; Elnar, A.A.; El Boustany, C. Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 2020, 27, 696–705.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277.
- Bhatt, B.; Zeng, P.; Zhu, H.; Sivaprakasam, S.; Li, S.; Xiao, H.; Dong, L.; Shiao, P.; Kolhe, R.; Patel, N.; et al. Gpr109a Limits Microbiota-Induced IL-23 Production To Constrain ILC3-Mediated Colonic Inflammation. J. Immunol. 2018, 200, 2905–2914.
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93.
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy 2011, 2011, 869647.
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855.
- Vallvé-Juanico, J.; Santamaria, X.; Vo, K.C.; Houshdaran, S.; Giudice, L.C. Macrophages display proinflammatory phenotypes in the eutopic endometrium of women with endometriosis with relevance to an infectious etiology of the disease. Fertil. Steril. 2019, 112, 1118–1128.
- Chadchan, S.B.; Popli, P.; Ambati, C.R.; Tycksen, E.; Han, S.J.; Bulun, S.E.; Putluri, N.; Biest, S.W.; Kommagani, R. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4, e202101224.
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12.
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic. mSystems 2019, 4, e00017-19.
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations between Endometriosis and Gut Microbiota. Reprod. Sci. 2021, 28, 2367–2377.
- Lüll, K.; Arffman, R.K.; Sola-Leyva, A.; Molina, N.M.; Aasmets, O.; Herzig, K.H.; Plaza-Díaz, J.; Franks, S.; Morin-Papunen, L.; Tapanainen, J.S.; et al. The Gut Microbiome in Polycystic Ovary Syndrome and Its Association with Metabolic Traits. J. Clin. Endocrinol. Metab. 2021, 106, 858–871.
This entry is offline, you can click here to edit this entry!
